INTRODUCTION
The Enhanced Recovery After Surgery (ERAS) protocol is a multimodal perioperative care pathway, designed to allow early recovery of patients who have undergone major surgery. The ERAS protocol has been shown to reduce the duration of hospital stay and incidence of complications, including ileus, as well as increase in the survival rate [
1]. However, it is difficult to apply an ERAS protocol as it requires the commitment of all members of the perioperative team and appropriate support from the staff of a large-scale institution. Moreover, developing, applying, and establishing the ERAS protocol is time- and effort-intensive. Other limitations include the need for patient education and a lack of social and cultural settings. Although various hospitals and departments have established and applied an ERAS system, few studies have described the overall ERAS setup process, including the development process, the team approach for ERAS application, and the establishment of the hospital-level system [
1].
This paper describes the process of developing and applying an ERAS protocol for colorectal cancer surgery through a team approach using a multidimensional method. Furthermore, we discuss the improvement process, compliance checks, and results of the program implementation.
Go to :

METHODS
In this study, all individual information was anonymized before data processing in order to comply with the privacy guidelines of the Health Insurance Portability and Accountability Act in Korea. The study protocol was approved by the Institutional Review Board of The Catholic University of Korea, Seoul St. Mary Hospital in Seoul, Korea (No. KC20RASI0859), which waived the necessity for informed consent based on the retrospective cohort design. This study was conducted in accordance with the ethical principles of the Declaration of Helsinki.
Implementation
In June 2009, good outcomes were observed by applying the ‘FAST track’, including early feeding, early ambulation, and early gastrointestinal motility medication on the first postoperative day (POD). FAST track was designed by colorectal surgery department of our hospital, and a few items of ERAS guideline were applied experimentally. However, the program was ceased due to the absence of essential personnel, including coordinators required for ERAS establishment, and lack of academic interest in the effects of ERAS implementation. Subsequently, in September 2016, under the leadership of the performance improvement team, 5 meetings were held with 26 multidisciplinary team members in attendance, including surgeons, anesthesiologists, project coordinators, ward nurses, nutritionists, and sports therapists administrative staff in insurance, and engineers, at the suggestion of the Division of Colorectal Surgery at Seoul St. Mary’s Hospital. In the first meeting, the members shared concepts and were presented with an ERAS-related thesis on colon cancer surgery to apprise them of the latest knowledge. The members discussed the means of applying the protocol in hospitals and the necessity of collecting previously missing anesthesia-related items (preoperative fasting period, epidural anesthesia, intraoperative fluid restriction, pain control). From the 2nd to 4th meeting, suitable protocols and education tools were developed, revised, and supplemented, with the insurance team confirming their implementation. During the 5th meeting, the clinical pathway (CP), patient education materials, and compliance measurement methods were completed. In October 2016, a pilot study was initiated with the application of a standard prescription (an enteral nutrition formula with a bowel preparation and a carbohydrate drink [NO-NPO, Yungjin Pharma, Seoul, Korea] at 2 preoperative hours) to patients with early cancer (American Society of Anesthesiology physical status classification, I; age, <60 years). Based on the results, the ERAS protocol was applied to all patients with colon cancer from January 2017, with continuous upgrades based on continuous monitoring and feedback. A rectal cancer ERAS protocol was developed in May 2017 and implemented in November 2017 (
Fig. 1).
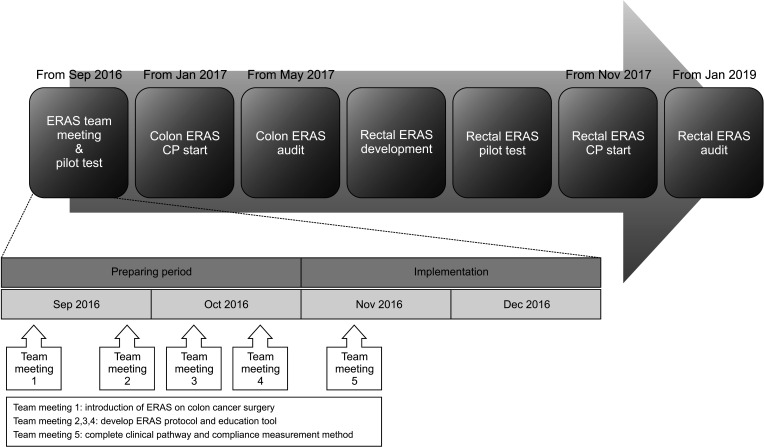 | Fig. 1The setup process for Enhanced Recovery After Surgery (ERAS) protocol. CP, clinical pathway.
|
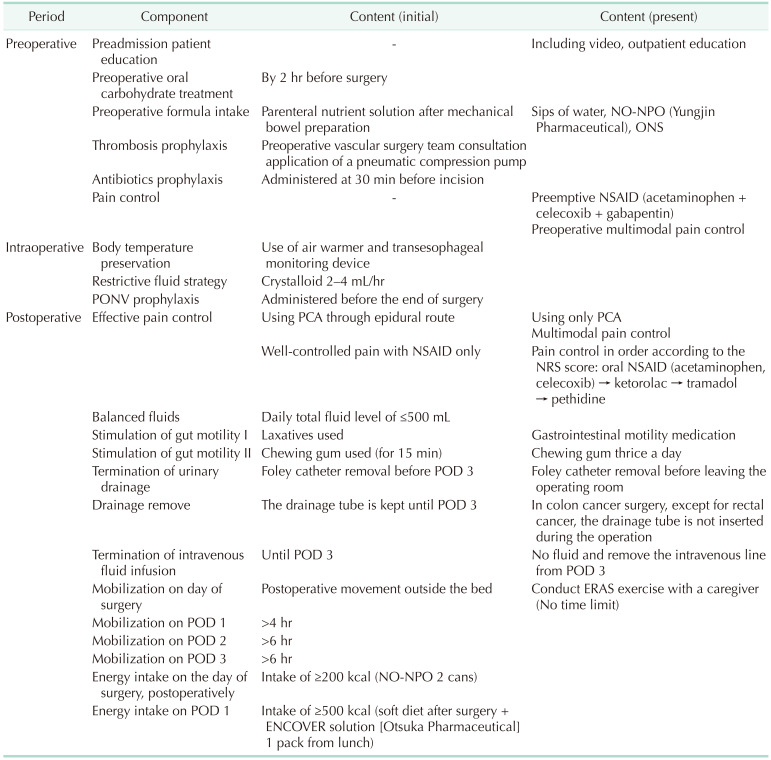
The initial ERAS protocol comprised 22 items divided into 3 phases: preoperative, intraoperative, and postoperative (
Table 1). To improve the protocol of the postoperative management of patients with colon cancer, surgeons and anesthesiologists collaborated from December 2016 to add items including epidural anesthesia, early feeding, and mobilization.
Table 1
Colon cancer ERAS protocol of our institution

Roles according to profession
Surgeon: The patients received explanations from the surgeon regarding the ERAS protocol and its perioperative role. First, preoperative education was provided to patients and their caregivers on an outpatient basis. In Korea, many patients do not want early postoperative discharge due to insurance coverage; therefore, we established accurate discharge protocol standards in advance and explained these to patients before hospitalization to ensure patients understood the parameters which enabled early discharge. Additionally, in our hospital, patients undergoing colorectal cancer surgery are usually admitted in the evening 2 days before the planned procedure to allow for appropriate surgery preparations. Therefore, patients who underwent surgery on Monday were hospitalized on the weekend; accordingly, they had reduced compliance with preoperative counseling and ERAS education. In these cases, the nurses reinforced patient education before hospitalization. A surgical team including the surgeon visited the operated patients twice a day to encourage early mobilization and oral feeding after surgery. CRP levels were checked before making a decision to discharge the patients.
Anesthesiologists: Before applying the ERAS protocol, the anesthesiologists were concerned regarding fasting for 2 hours before surgery due to the risk of aspiration during operation (in our center, standard fasting time before surgery under general anesthesia is 8 hours for solid, 2 hours for liquid except for water); however, they approved this in a meeting prior to ERAS initiation. Initially, the 2-hour fast was implemented for patients with colon cancer with advanced consultations on an outpatient basis; subsequently, the scope of the practice was expanded. Intraoperatively, anesthesiologists managed the following: fluid restriction; blood pressure, use of inotropic drugs if necessary; and hypothermia prevention. Additionally, an anesthesiologist performed perioperative pain control; moreover, epidural anesthesia was explained to the patient providing consent on an outpatient basis before hospitalization. Postoperatively, patient-controlled analgesia (PCA) was provided, and the pain score was checked. Additional pain control intervention was performed for patients with uncontrolled pain.
Ward nurse: After hospitalization, the ward nurse supplemented insufficient education about ERAS before surgery; further, they checked the amount of food consumed during the early postoperative oral feeding period in the ward. Additionally, they checked the patients’ exercise time, exercise distance, and calories burned.
ERAS managing nurse: An ERAS managing nurse was in charge of perioperative ERAS education and performance management and also reviewed the literature and guidelines. Additionally, the nurse reviewed the patient’s perioperative body composition using bioelectrical impedance analysis, referred patients to the nutrition team for nutritional counseling and reinforcement, and Department of Rehabilitation Medicine for postoperative exercise therapy if sarcopenia was present.
Nutritionist: The nutritionist explained the concept of early oral feeding, provided perioperative nutrition education, and encouraged preoperative oral nutrition drinks and enteral nutrition.
Project coordinator: The project coordinators planned and organized the meetings. Additionally, they facilitated communication between the doctors, nurses, and patients, to help resolve any problems that arose during the protocol application.
Computer team: The computer team built the ERAS CP (standard treatment guideline).
Improvement of protocol
After applying the first ERAS protocol, ERAS compliance was monitored and audited every 4–6 months. The first meeting after ERAS implementation was in May 2017. Multidisciplinary team members gathered again to share the outcomes and experiences of applying the protocol to 50 patients with colon cancer from January to April 2017. In this meeting, the compliance measurement method was modified. The compliance rates for each specific ERAS protocol item by year are summarized in
Tables 2 and
3. The compliance rate was negatively correlated with the patient’s recovery and positively correlated with the duration of hospital stay. Therefore, in 2017, the surgeon conducted a journal review and a compliance check and discussed the plans for protocol improvement. Improved items included patient education, bowel preparation, and perioperative fluid restriction. If possible, patient education was provided on an outpatient basis; further, patient compliance was improved by adjusting the time for performing preoperative endoscopy and bowel preparation. Additionally, a vasopressor was intraoperatively used to prevent blood pressure decrease due to fluid restriction. Moreover, the initial protocol was modified to include the application of a Foley catheter and drainage tube. The Foley catheter was removed before POD 3 in the initial protocol; however, it was removed immediately after surgery after the protocol review. Further, the drainage tube was initially removed on POD 3; however, after 2019, it was not initially placed for patients with colon cancer.
Table 1 summarizes the details of both protocols.
Table 2
Compliance rate of colon cancer Enhanced Recovery After Surgery protocol (n = 602)
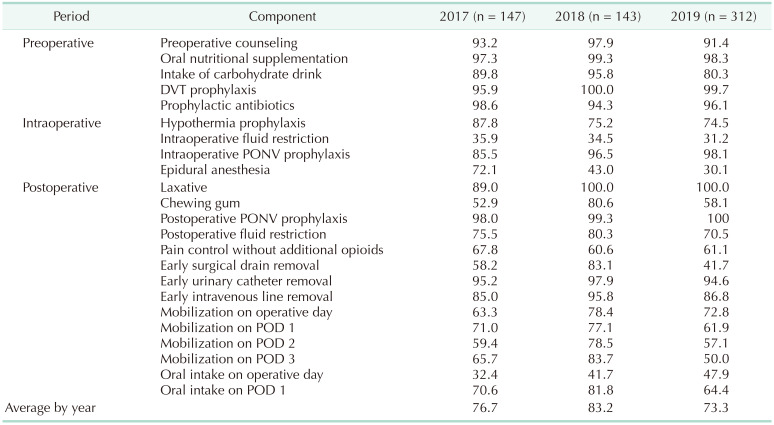

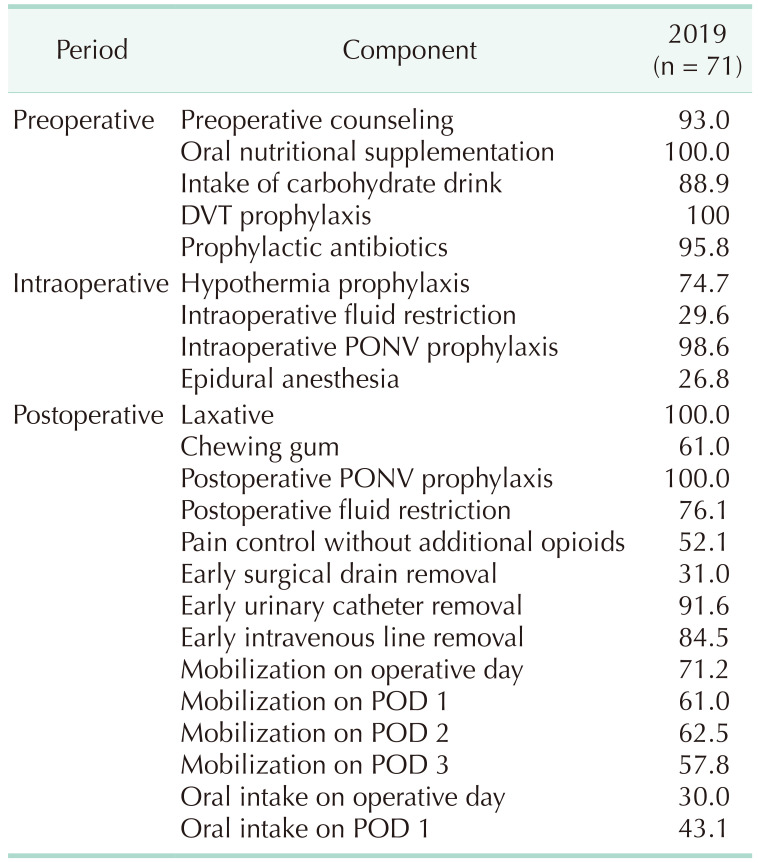
Table 3
Compliance rate of rectal cancer Enhanced Recovery After Surgery protocol

The authors have previously published papers on ERAS protocol implementation and presented them to the Quality Improvement Society, Colon and Anal Society, Society for Surgical Metabolism and Nutrition, Korean Society for Parenteral and Enteral Nutrition, ERAS Society, and the Society for Surgical Infectious Diseases. These papers included the comparison of inflammation [
2], surgical site infection [
3], and complication types before and after protocol application [
45]; comparisons of systemic and technical approaches [
4]; and a comparison of compliance with the ERAS system [
4]. Previous analyses found that applying the protocol decreased the inflammation and shortened the duration of hospital stay, without any difference in the complication rates; moreover, there was adequate postoperative pain control. The epidural anesthesia was applied for pain control when the ERAS protocol was initiated. However, after continuous monitoring and discussion with an anesthesiologist, the effect was found to be low and it was excluded from the standard prescription. Since September 2019, multimodal pain control was administered using intravenous PCA and oral NSAIDs.
A study showed that cancer patients with preoperative sarcopenia had a longer duration of hospital stay than those without; moreover, they had a poor postoperative recovery rate and prognosis. Based on these results, a body composition measurement tool such as a body composition analyzer (InBody Co., Ltd., Seoul, Korea) was used to perioperatively check for sarcopenia; further, the protocol could be continuously upgraded by predicting the change and prognosis of the patient through serial measurements. Additionally, studies on prehabilitation, which is increasingly important in the perioperative recovery process, are currently in progress. In our hospital, prehabilitation was introduced into the ERAS protocol through collaboration among various clinical departments, and its monitoring is ongoing.
ERAS allowed a reduction in the duration of hospital stay and improvements in patient satisfaction with continuous monitoring and improvement of the protocol. These achievements helped in gaining the hospital's support and establishing ERAS as a hospital-level system. Additionally, national and Joint Commission International (JCI) certifications were obtained, which further established that the ERAS system of colorectal surgery in our hospital provides a high standard of care. Moreover, external lectures and press announcements were made to publicize the advantages of the ERAS system of our hospital.
Statistical analysis
The length of hospital stay after application of colon cancer ERAS protocol in 2017, 2018, and 2019 were summarized as mean ± standard deviation (SD) and compared using the 1-way analysis of variance (ANOVA). The rectal cancer ERAS protocol was applied since December 2017, so the lengths of hospital stay in 2018 and 2019 were expressed as mean ± SD and compared using the Student t-test. These contents are summarized in
Table 4. Also, complication rate and severe complication rate after application of colon cancer ERAS protocol in 2017, 2018, and 2019 were compared using the chi-square test. The contents are summarized in
Table 5. Although the rectal cancer ERAS protocol has been applied since December 2017, the audit was performed from 2019. So only the changes in 2019 were expressed as graphs (
Fig. 2C, D). Finally, the patient compliance rate of colon cancer ERAS protocol in 2017, 2018, and 2019 were summarized as mean ± SD and compared using the 1-way ANOVA. As with the complication rate, the audit of rectal cancer ERAS protocol was performed from 2019. So only the changes in 2019 were expressed as a graph (
Fig. 3). Statistical significance was set at P < 0.05 and statistical analyses were performed using IBM SPSS Statistics ver. 24.0 (IBM Corp., Armonk, NY, USA).
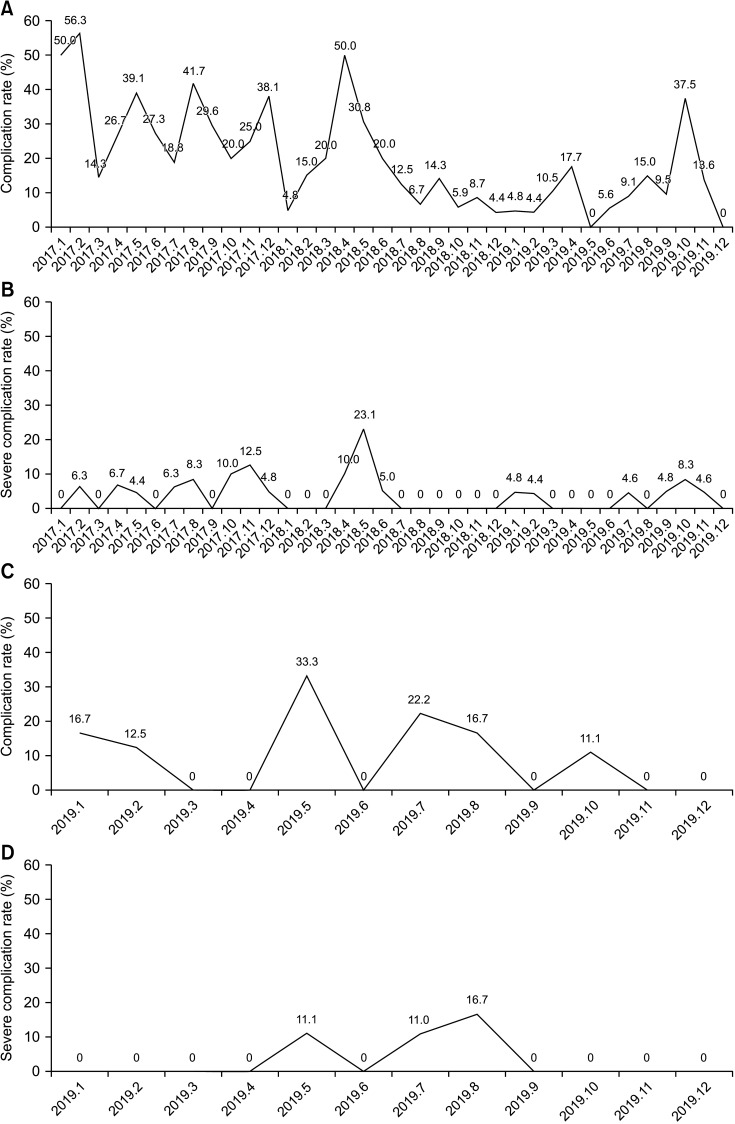 | Fig. 2(A, B) Complication rates (A) and severe complication rates (B) for colon cancer surgery after application of the Enhanced Recovery After Surgery (ERAS) protocol. (C, D) Complication rates (C) and severe complication rate (D) for rectal cancer surgery after application of the ERAS protocol.
|
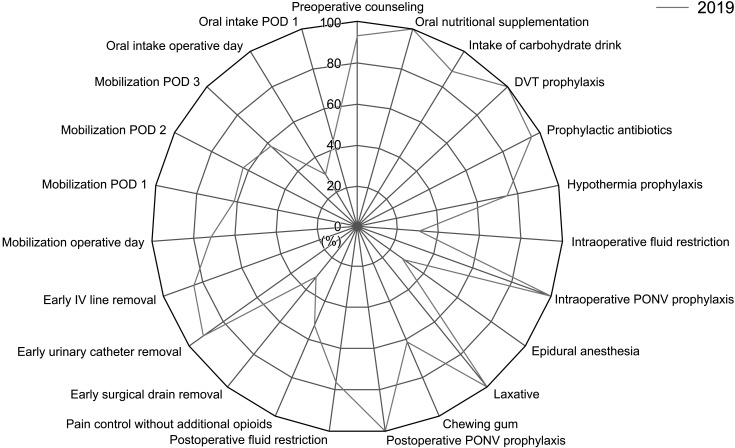 | Fig. 3Compliance rates of rectal cancer Enhanced Recovery After Surgery protocol. DVT, deep venous thrombosis; PONV, postoperative nausea and vomiting; IV, intravenous; POD, postoperative day.
|
Table 4
Length of hospital stay (LOS) after application of colon and rectal cancer Enhanced Recovery After Surgery protocol


Table 5
Complication and severe complication rate after application of colon and rectal cancer Enhanced Recovery After Surgery protocol

Go to :

RESULTS
After application of the colon cancer ERAS protocol in January 2017, there was a significant decrease in the length of hospital stay between 2017 and 2018 (5.6 ± 3.2 days [2017], 4.6 ± 1.5 days [2018], and 5.3 ± 5.0 days [2019]; 2017–2018, P = 0.005 and 2018–2019, P = 0.129) (
Table 4,
Fig. 4). There was no significant difference in the duration of hospital stay after applying the rectal cancer ERAS protocol (4.9 ± 1.2 days [2018], 5.2 ± 2.4 days [2019]; P = 0.740) (
Table 4,
Fig. 5). There was a significant decrease in complication rate from 2017 to 2019 after initiation of the colon cancer ERAS protocol (
Table 5,
Fig. 2A). And there was no statistically significant difference in the severe complication rate (Clavien-Dindo classification, ≥III) for 3 years (
Table 5,
Fig. 2B). In the case of rectal cancer, the protocol was applied from December 2017, but the audit started from 2019. In 2019, after rectal cancer ERAS protocol was applied, the complication rate was steadily decreasing, and the severe complication rate also showed the same trend (
Fig. 2C, D). Since January 2017, there was an increase in the ERAS CP application rate over time, even after November 2017, when the rectal cancer ERAS CP was also applied. After December, there was a plateau at a rate of >90% (
Fig. 6). The compliance rates for the colon cancer ERAS protocol were 76.71% ± 11.91%, 83.24% ± 9.31%, and 73.33% ± 11.33% in 2017, 2018, and 2019, respectively. In 2018, compliance rate increased significantly compared to 2017; but in 2019, the rate showed a significant decrease compared to 2018 (2017–2018, P < 0.001; 2018–2019, P < 0.001) (
Table 2,
Fig. 7). And the compliance data of rectal cancer ERAS protocol was also collected from 2019, the compliance rate for each item of the rectal cancer ERAS protocol in 2019 showed an overall similar trend to that of colon cancer ERAS protocol (
Table 3,
Fig. 3).
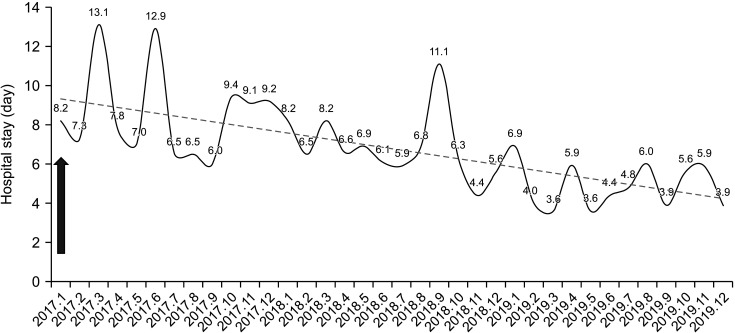 | Fig. 4Hospital stay of colon cancer surgery. Arrow indicates the start time of colon cancer Enhanced Recovery After Surgery (January 2017).
|
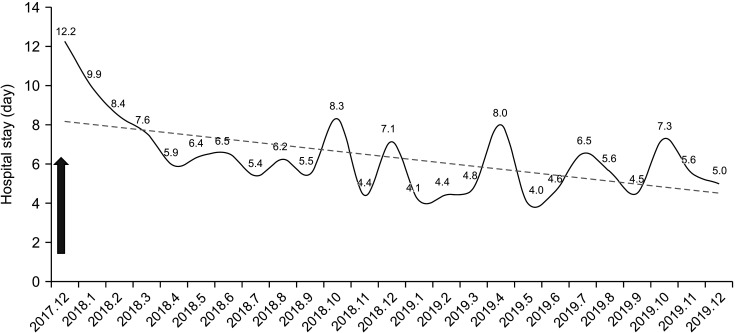 | Fig. 5Hospital stay of rectal cancer surgery. Arrow indicates the start time of rectal cancer Enhanced Recovery After Surgery (December 2017).
|
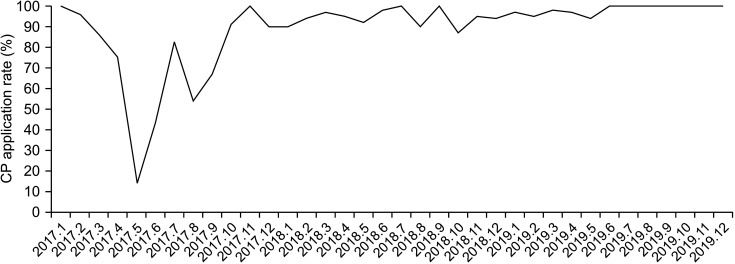 | Fig. 6Colorectal cancer Enhanced Recovery After Surgery clinical pathway (CP) application rates.
|
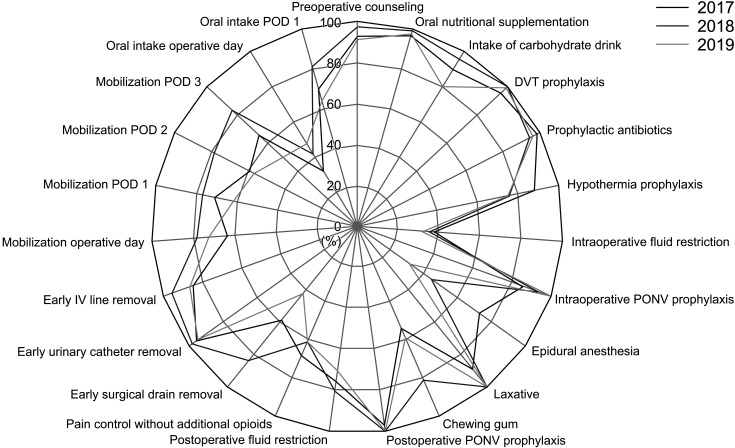 | Fig. 7Compliance rates of colon cancer Enhanced Recovery After Surgery protocol. DVT, deep venous thrombosis; PONV, postoperative nausea and vomiting; IV, intravenous; POD, postoperative day.
|
Go to :

DISCUSSION
Since the ERAS protocol was first introduced over 30 years ago, its effectiveness has been recognized in various surgical fields, including colorectal surgery [
6]. However, it is not easy to challenge the existing doctrine; moreover, there remain limitations to its application, given the different circumstances of each hospital.
For successful development and application of the ERAS protocol, a multidisciplinary team approach, including a performance improvement team, is essential; moreover, the purpose of applying ERAS and the goal of the team leader must be clear [
7]. We learned the concept of ERAS; determined the roles for each practitioner; and developed patient education materials, the standard prescription, CP, and compliance measurement tools through discussions in 5 meetings before implementing the ERAS system in our hospital. From the beginning, every professional staff member attended the meetings to ensure a team approach. The main reason for the failure of the previous (2009) program was difficulties with reaching agreements in the multidisciplinary team setting. Therefore, thorough prearrangements were made in advance to support the success of the new program. After this process, a pilot study was started in October 2016 for patients with colon cancer who were young and in good general condition, which confirmed the compliance data and problems when applied to actual clinical practice. Moreover, a monitoring tool was developed to conduct audits and compliance checks. Since January 2017, the ERAS protocol has been applied to all patients with colon cancer.
One of the most important steps after applying an ERAS protocol is checking the compliance of each protocol item and reinforcing patients, which is difficult in actual clinical practice [
8]. In our hospital, compliance audits were regularly conducted after protocol application to strengthen the compliance for each item. For example, at first, Foley catheter and surgical drain were removed 3 days after surgery. However, according to the monitoring result, it was changed to be removed one day after operation, and compliance rate seemed to decrease in 2019. But positive outcomes were obtained in that there was a significant decrease in the duration of hospital stay after application of the ERAS protocol.
However, there remain some areas for improvement in compliance checks. First, the compliance check takes a long time and is not objective, since it depends on the patient’s feedback. In addition, since the results of the compliance check cannot be conducted in real-time, the situation is usually estimated by reviewing retrospective data. Therefore, there is a limitation in that compliance check cannot be completed to apply its findings immediately for patient care. Furthermore, extensive research is required to improve this. A study conducted by Rauwerdink et al. [
9] in 2019 showed an increased compliance for postoperative mobilization among patients using a patient-centered mobile application. Therefore, patient involvement in the ERAS system through a mobile phone application could ultimately facilitate fast recovery from surgery. In our hospital, using a wearable device in the ward, the patient’s steps, distance exercised, and calories consumed were measured in real-time to check for items with poor compliance. Accordingly, patients were reinforced to significantly improve compliance. We are currently developing a mobile application for patient-users, which will allow for more effective compliance management.
Additionally, some of the items of the ERAS protocol implemented in our hospital differed from those in the original ERAS guidelines, including bowel preparation and drainage tube insertion. The reasons for using bowel preparation are explained in the guidelines; however, there remains disagreement regarding combining bowel preparation with antibiotics [
10]. Based on the 2018 ERAS guidelines, there is no clinical advantage if bowel preparation is performed without antibiotics, but it may cause dehydration and discomfort in colon cancer; however, it is helpful in rectal cancer [
10]. Additionally, combining oral antibiotics and mechanical bowel preparation reduced postoperative morbidity compared with bowel preparation alone. This differs from the pre-2018 guidelines that recommended bowel preparation alone [
11]. Currently, patients with ascending colon cancer do not undergo bowel preparation; contrastingly, patients with other colon cancers and rectal cancer receive oral nutrition supplementation, fluid intake, and oral carbohydrate liquid after bowel preparation. Currently, there is ongoing research on the range of application of bowel preparation and the application method and range may differ in the future depending on those findings.
Regarding drainage tube insertion, the original ERAS guidelines do not recommend routine drainage tube insertion in patients with colorectal cancer, since it does not reduce anastomotic leakage, mortality, wound infection, or reoperation rate [
11]. At the initial stages of implementation of the ERAS system, the drainage tube was removed on POD 3 in cases with no specific problems. Subsequently, the protocol was modified to avoid insertion in colon cancer surgery.
Several hospitals worldwide have reported that applying the ERAS protocol decreased the duration of hospital stay with no change in the complication rate and a few other hospitals reported a reduced complication rate [
1213]. However, given the reduced hospital stay, some patients complain about being discharged early. Especially in Korea, patients often want a longer hospitalization period because the amount of insurance can increase depending on the length of hospital stay. To prevent this, our medical staff explained the ERAS protocol in the outpatient clinic before admission and repeatedly provided education during hospitalization. Moreover, we checked the CRP level before discharge and deferred the discharge if it was ≥9 mg/dL. The Charlson comorbidity index (CCI) score, which was applied in the improvement process, assesses several items regarding anastomosis-related complications, digestive system, respiratory system, cardiovascular system, and surgical wounds [
14]. The score was used to comprehensively predict the patients’ postdischarge conditions and complications. From 2018, the criteria for this CCI score were further subdivided to strengthen the evaluation standard, which could have attributed to the increase in the complication rate in 2019. Since 2018, the ERAS CP application rate has plateaued at approximately 100%. Therefore, there is a need for further research to compare the complication rate before and after subdividing the CCI score in a center with an established and stable ERAS protocol.
The ERAS system is a program that allows quick postoperative recovery. In this program, it is important to set goals based on standard criteria and change them based on the circumstances of each hospital. This requires a team with sufficient understanding and knowledge of ERAS protocols; moreover, thorough role allocation, collaboration, and education should be implemented for each job within the team. Additionally, continuous correction and supplementation should be performed through continuous monitoring and academic research after implementing the ERAS. Better results can be expected with monitoring of the major factors affecting the patient compliance; moreover, items with low compliance can be reinforced quickly through real-time monitoring and continuous communication with the patient.
Go to :








 PDF
PDF Citation
Citation Print
Print












 XML Download
XML Download