Abstract
Purpose
Cholecystectomy is one of the most common surgeries today due to gallbladder diseases. The most prevalent malignancy of the biliary tract is gallbladder cancer. We aimed to discuss the results of our patients who underwent cholecystectomy for benign reasons in our clinic and who had gallbladder cancer due to pathology.
Methods
The results of cholecystectomy performed in General Surgery Clinic of Seyhan Government Hospital were evaluated. Cases diagnosed as gallbladder as a result of histopathological examination were included. Preoperative ultrasonography, laboratory findings, and postoperative pathology results of the patients were reviewed retrospectively. The pathologist repeated histopathological evaluations.
Results
Between 2010 and 2019, incidental gallbladder cancer (IGBC) was detected in 40 patients (0.3%) in 11,680 cholecystectomy operations. Of the patients diagnosed with IGBC, 14 (35.0%) were T1a, 11 (27.5%) were T1b, 11 (27.5%) were T2, and 4 (10.0%) were T3. T4 tumor was not seen in any patient. Three patients who were T1b at initial evaluation were identified as T2 at evaluation for the study. The pathology results of 37 patients (92.5%) were adenocarcinoma, 2 (5.0%) were adenosquamous type, and 1 (0.5%) was squamous cell carcinoma.
Conclusion
There has been a remarkable increase in the number of IGBCs over the past 20 years. Appropriate staging and histopathological evaluation are essential in guiding the surgeon’s operation. It is crucial to accurately determine the T stage, the most influential parameter on patient survival and residual recurrences. The distinction between pathologic (p) T1a and pT1b should be made carefully. Surgery is the only potentially curative method.
Gallbladder cancer (GBC) is the most common cancer of the biliary system and the fifth most common cancer of the gastrointestinal tract [1]. The diagnosis of cancer as a result of examination of pathology specimens of patients who were operated on with a preliminary diagnosis of benign gallbladder diseases is called incidental GBC (IGBC). Elderly, female patients and conversion from laparoscopic surgery to open surgery are stated as risk factors for IGBC [2]. IGBC accounts for 50%–70% of all newly diagnosed GBCs [34]. Increased wall thickness, irregularities of the mucosa and submucosa, and an increase in echo are all suspicious findings for malignancy on ultrasonography (USG), which plays an important role in diagnosis [5]. IGBCs are usually early-stage cancers [6]. The most effective treatment of IGBC is surgery. In histopathological evaluations, pathologic (p) T stage and presence of lymphatic involvement determine patient survival along with the surgical or medical approach to be applied in the treatment [3]. In IGBC, the pT stage is the most critical factor in deciding on the second surgical intervention [7]. Cholecystectomy is sufficient for pT1a invasion depth. Extended hepatic resection and regional lymphadenectomy are recommended for patients with pT1b and greater depth of invasion due to its positive contribution to survival [8]. A residual tumor is detected in approximately 60% of patients who undergo re-resection after the first surgery; with an increase in T stage, the probability of encountering a residual tumor increases [910]. Controversy continues regarding the role, timing, extent, and impact of secondary surgery on survival in IGBC. With correct staging and appropriate surgical strategy, a 5-year survival rate can be up to 70% in pT1–2 patients [4]. On the other hand, despite all the advances in surgical and medical oncological treatment, 5-year survival in patients with lymphatic involvement or metastatic disease is below 25% [11]. The aim of our study is to evaluate the clinical characteristics, surgical approaches, and pathology results of patients diagnosed with IGBC.
Study protocol approval was received from the Institution Review Board of Adana City Hospital on September 23, 2020 (No. 66-1071). Written consent was obtained from the patients for the use of surgical images.
The data of 11,680 patients who were operated on with the preliminary diagnosis of benign gallbladder diseases between January 2010 and September 2019 in the General Surgery Clinic of Seyhan Government Hospital were assessed using an electronic database. Patients with a preliminary diagnosis of GBC (n = 11) and gallbladder polyp (n = 6) were excluded from the study. Forty patients diagnosed with IGBC, who underwent emergency and elective cholecystectomy for benign reasons, independent of surgical technique, were included in the study. The patients’ sex, age, preoperative laboratory results, imaging reports, surgery reports, pathology results, postoperative follow-ups, and information about the second surgery were recorded. In the evaluation of the patients using USG, the presence of stones, stone size, and wall thickness information were examined. Gallbladder stone diameters greater or less than 3 cm and gallbladder wall thickness of more than or less than 3 mm were evaluated. Also, the blocks and the slides of these cases were reevaluated by the pathologist. Carcinoma type, histologic grade, depth of invasion, surgical margin, and perineural and lymphovascular invasion status of the tumors were reexamined. Some of the blocks and/or slides of the first evaluation could not be found; the information stated in the pathology report was taken into account.
TNM classification was made with the histopathological examinations of the patients and the radiological evaluations made after diagnosis. It was observed that T1a patients did not undergo a second surgical intervention. Triphasic abdominal computed tomography examinations performed before re-excision were evaluated in patients with T1b and more advanced pT tumors. Second surgical interventions were performed 6–10 weeks after the first operation. Preoperative trocar site recurrence, intraperitoneal, and metastatic spread in the liver capsule were studied in the medical records. Complications that developed within the first 30 days after surgery were recorded. Complications were classified and evaluated according to the Clavien-Dindo classification of surgical complications.
IBM SPSS Statistics ver. 23.0 (IBM Corp., Armonk, NY, USA) was used for statistical analysis of the data. Categorical measurements were summarized as numbers and percentages, and continuous measurements as mean, standard deviation, and median (interquartile range [IQR]). The variables’ suitability to normal distribution was examined using visual (histogram and probability graphics) and analytical methods (Kolmogorov-Smirnov/Shapiro-Wilk tests). The chi-square test was used in comparing categorical variables. One-way analysis of variance was used for parameters conforming to the normal distribution, and the Kruskal-Wallis test was used in groups that did not comply with a normal distribution. Kaplan-Meier analysis, log-rank tests, and Cox regression analysis were used for survival analysis. In all tests, the statistical significance level was set at 0.05.
Of the 11,680 patients included in the study, 3,540 (30.3%) were male, 8,140 (69.7%) were female, and the mean age was 52.4 years (18–95 years). Cholelithiasis was present in 10,830 patients (92.7%) who underwent cholecystectomy. IGBC was detected in 40 of the patients (0.3%) who underwent cholecystectomy after histopathological examination. Of these patients, 11 (27.5%) were male and 29 (72.5%) were female. The mean age of the patients diagnosed with IGBC was 62.3 years (IQR, 38–82 years). Demographic and clinical characteristics of the patients are presented in Table 1.
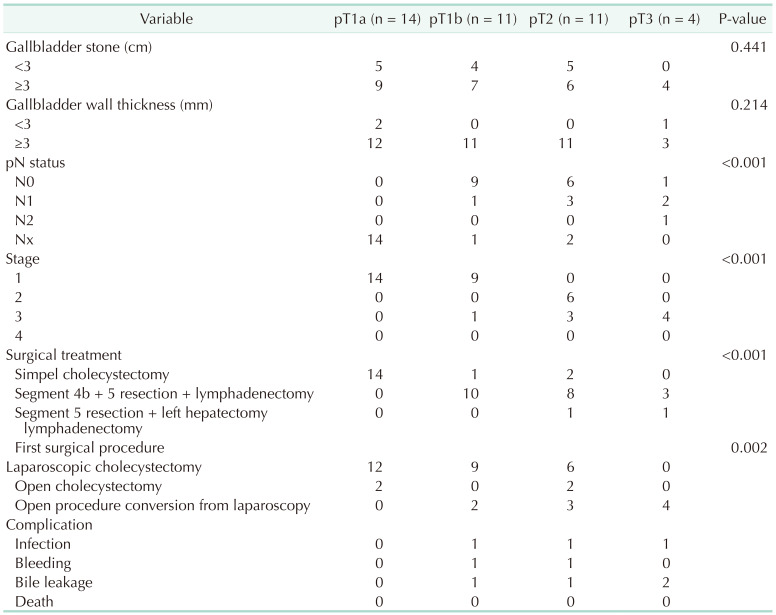
All patients with IGBC were found to have gallstones on USG. When the association of gallstones in 11,680 patients who underwent cholecystectomy and IGBC patients was evaluated, the presence of stones in IGBC was found to be statistically significantly higher (P = 0.049). The size of gallstones was smaller than 3 cm in 14 patients and larger than 3 cm in 26 patients. Gallbladder stones larger than 3 cm had no statistically significant effect on the advancement of the pT stage (P = 0.441). The gallbladder wall thickness was greater than 3 mm in 37 individuals, and less than 3 mm in 3 cases, as per radiological evaluation. No significant correlation was observed between the increase in wall thickness and progression in pT stage (P = 0.214). Similarly, no effect was observed on the presence of tumor at the surgical margin (P = 0.850).
Cholecystectomy was performed urgently in 4 patients due to acute cholecystitis and in 36 patients in elective conditions. Surgery was started with laparoscopic surgery in 36 of the patients and open method in 4 patients due to previous abdominal surgery. In 9 patients who were started laparoscopically, cholecystectomy was converted to open surgery due to the inability to perform safe cholecystectomy and advanced adhesion. It was observed that the pT stage of IGBC was effective on conversion cholecystectomy (P < 0.001).
In the evaluation made according to the depth of tumor invasion, it was determined that 12 of the patients who underwent laparoscopic cholecystectomy were T1a, 9 were T1b, and 6 were T2. Of the patients who underwent open cholecystectomy, 2 were at stage T1a and 2 were at stage T2. In the pathological outcome of the patients who converted from laparoscopic procedure to open surgery, 2 were determined as T1b, 3 as T2, and 4 as T3. As a result of this evaluation, there was an increase in the rate of conversion from laparoscopy to open surgery with an increase in the depth of tumor invasion. This increase was statistically significant (P = 0.002). Perforation of the gallbladder occurred in 3 patients. Two of these patients were pT1a and 1 was pT3. Open surgery was performed in the patient with pT3. The perforated gallbladders were removed from the abdomen with an endo bag, according to the findings. It was determined that T stage (P = 0.530) and stone diameter (P = 0.630) had no effect on gallbladder perforation. In all patients, only cholecystectomy was performed as a standard in their first surgery.
Patients with T1a did not require a second operation following cholecystectomy, according to the findings. It was observed that 1 patient with T1b and 2 patients with T2 did not undergo extended surgical resection due to additional diseases, high American Society of Anesthesiologists physical status classification, and the patient’s refusal to accept additional surgical intervention. More radical surgeries were performed due to the increase of the patients in T stage (P < 0.001) (Tables 2 and 3).
Tumor-free surgical margin was achieved in all patients who underwent liver resection and lymph node dissection after the diagnosis of IGBC. Tumor involvement was detected in the left lobe of the liver in 1 patient with pT3 and the diaphragm in 1 patient. In this patient, segments 4b and 5, and tumoral focus on the diaphragmatic area were resected and lymphadenectomy has been made (Fig. 1).
According to the histopathological reevaluation of the results, in all cases, 37 were adenocarcinoma, 2 were adenosquamous carcinoma, and only 1 case was squamous cell carcinoma. Biliary (45.0%) and intestinal types (40.0%) were the most common adenocarcinoma subtypes, while mucinous type and poorly cohesive type were present in 2 cases (5.0%) and 1 case (2.5%), respectively. Three cases were reported as T1b at the initial evaluation, and these patients were observed to be pT2 in the evaluation performed by the pathologist for this study. In the histopathological evaluation, more than half of the cases were grade 2 (52.5%), while lymphovascular and perineural invasion was observed in 27.5% and 40.0%, respectively (Table 2).
According to the Clavien-Dindo surgical complication classification, 3 patients were grade I, 5 patients were grade II, and 1 was grade III. There were no grade IV or V complications. Wound infection developed in 3 patients, which was treated with surgical drainage. Wound infection developed in 2 patients converted from laparoscopic surgery to open surgery in the first operation. All other complications occurred after the second surgery. Biliary fistula developed in 4 patients. Three patients were treated spontaneously with medical treatment and follow-up, and 1 patient was treated after endoscopic retrograde cholangiopancreatography. Bile duct resection was not performed in any patient due to biliary fistula. Bleeding from the liver resection line occurred in 2 patients. They were treated with blood transfusion and a conservative approach without any interventional procedure. The patients in this study experienced no mortality throughout the perioperative and early postoperative periods.
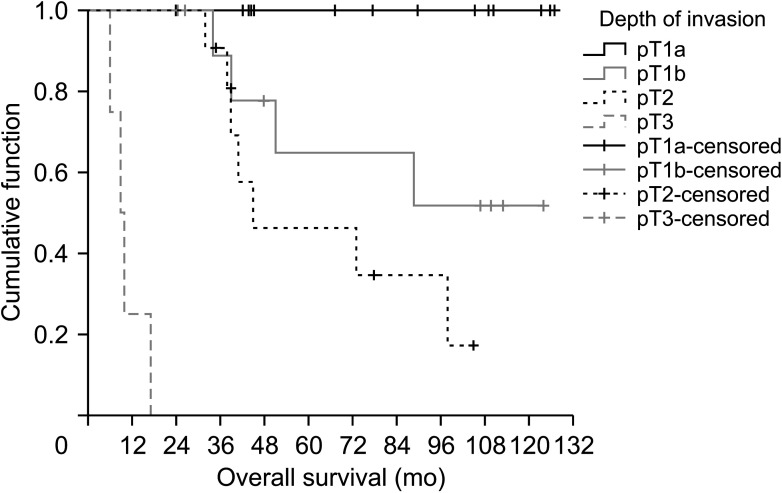
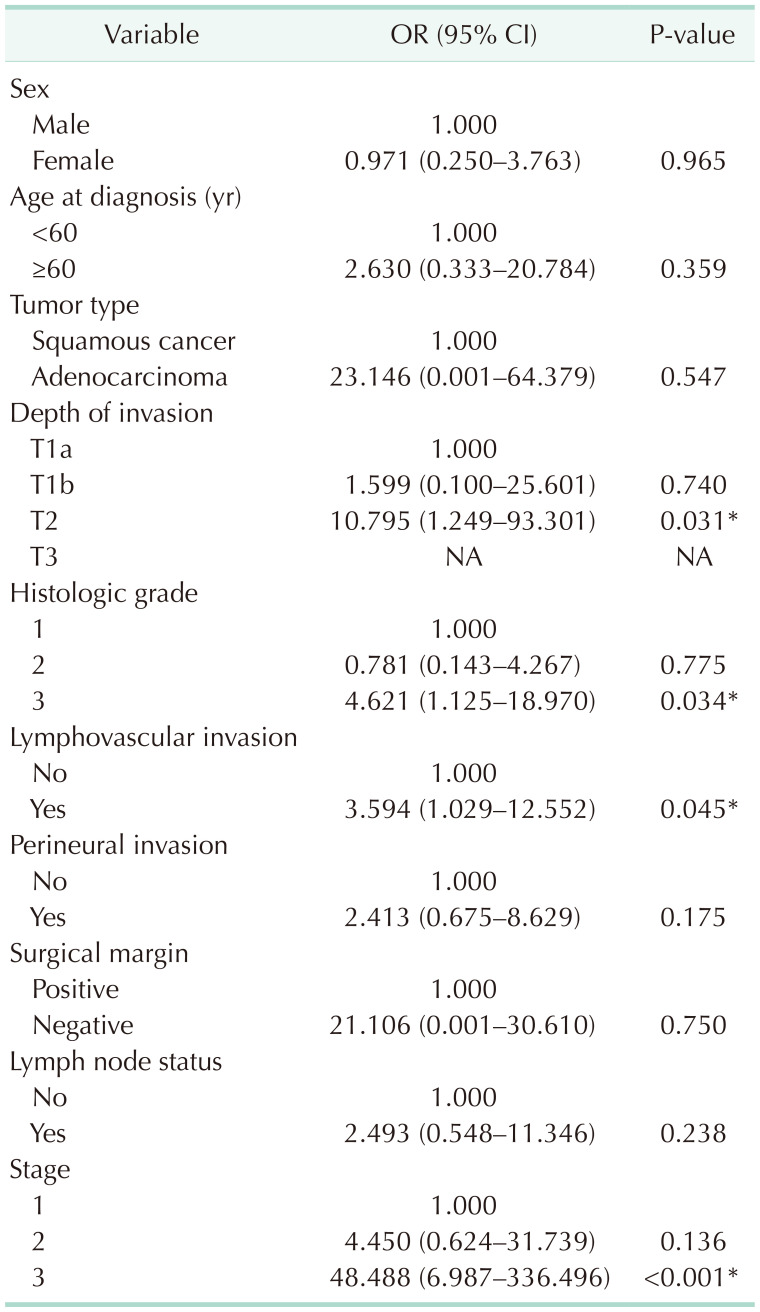
Finally, according to the depth of invasion, the patients’ survival data were compared using Kaplan-Meier and log-rank analysis. Based on depth of invasion, 5-year survival was 100% in pT1a, 45% in pT1b, 27% in pT2, and 0% in pT3 (Fig. 2). Overall survival was much lower with increasing depth of invasion (P < 0.001). When comparing the depth of invasion with the stage, it was determined that the progression in pT caused a statistically significant increase in the stage state. The median survival times and 5-year survival rates of the patients according to the T groups are given in Table 3. On the other hand, the effects of age at diagnosis, sex, tumor type, depth of invasion, histological grade, lymphovascular and perineural invasion, surgical margin, lymph node status, and stage on survival were analyzed by univariate Cox-regression analysis. It was determined that the depth of invasion, histological grade, lymphovascular invasion, and the increasing stage had a significant adverse effect on survival (Table 4).
Approximately 70% of GBCs are diagnosed after careful examination of the specimens of patients who were operated on for benign reasons [1]. Diagnosis of IGBC is detected more frequently in routine evaluations than in selective histopathological evaluations [12].
Chatelain et al. [13] reported that patients diagnosed with IGBC often have missing data in their reports for important prognostic factors, including tumor stage, size, grade, and resection margins. The surgical margin, histological grade, lymphovascular invasion, perineural invasion, pT, and lymph node involvement, all of which are crucial in the treatment and prognosis of patients diagnosed by the pathologist incidentally, should all be stated in the reports [1314]. In our study, although deficiencies in pT, surgical margin, and lymphatic node evaluation were not observed in pathology reports, partial deficiencies were determined in histological grade, perineural, and lymphovascular evaluations. The depth of tumor invasion in the samples of 3 individuals was revealed to be greater than the previous one in the second pathological examination. It was thought that it was due to an examination without taking enough samples.
The elderly and female sex are among the demographic risk factors for IGBCs. It is diagnosed 1.3–3.5 times more in women. GBCs are usually detected over the age of 65 years [14]. It was determined that IGBCs were higher in advanced age and female sex. While the GBC average age at diagnosis is 65 years and up, the IGBC average age appears to be a few years lower [15]. We observed that our patients had a mean age of 62.3 years, a few years lower than those reported in the literature.
Co-occurrence of cholelithiasis in patients diagnosed with IGBC has been reported as high as 94% in studies [16]. Multiple gallbladder stones and larger than 3-cm stones in USG are reported as factors that increase the risk for cancer [17]. Gallstones were detected on USG in all of the patients in the study. In 14 of 40 patients (35.0%), the diameter of the stones was 3 cm, and in 26 (65.0%), the stones were larger than 3 cm. Although the stone diameter was larger than 3 cm in the majority of the patients, contrary to the literature, the effect of stone size on the stage progression was not statistically significant.
The increased wall thickness of the gallbladder on USG is another risk factor for IGBC. Our study determined that the gallbladder wall thickness was more than 3 mm in 37 of our patients (92.5%). In our study, no statistically significant relationship was found between the increase in gallbladder wall thickness and the effect of tumor-free surgical margin resection and pT progression. Although IGBC wall thickness increase is an important finding, it was considered not a specific marker.
The increase in pT status in IGBC had a statistically significant effect on the rate of conversion cholecystectomy. Increased adhesion, fibrosis, tumor invasion, and previous inflammatory episodes related to gallstones were thought to cause conversion cholecystectomy more than cholecystectomy for benign reasons in individuals with IGBC. In addition to the increase in the size of stone and wall thickness of the gallbladder, it should be considered that there may be a tumor in gallbladder diseases that are difficult to complete laparoscopically or that are converted to open surgery.
In IGBC, intraoperative perforation of the gallbladder is associated with tumor recurrence at the trocar entry site, recurrence in the diaphragm, tumor implants on the liver surface, poor prognosis, failure to perform curative surgeries, and peritoneal carcinomatosis [18]. In this study, it was determined that pT did not affect the increase in perforation rates. In 1 of the 3 patients who developed intraoperative perforation, laparoscopic surgery was completed with open surgery. Trocar site recurrence did not occur in the follow-up of the other 2 patients. It was thought that removing the perforated gallbladder with the endo bag used in laparoscopic surgery might be effective. In the second operation of the patient who had gallbladder perforation and was completed open, tumor implantation was observed in an area of 3 × 4 cm on the diaphragmatic surface and was resected.
Simple cholecystectomy is sufficient in patients evaluated as T1a in the surgical treatment of IGBC. Liver resection and lymphadenectomy do not affect survival. The 5-year survival rate for pT1a tumors has been reported to be 90%–100% in studies [18]. In our study, no additional surgical intervention was performed for cholecystectomy in 14 patients with pT1a. Our 5-year survival rate was slightly less than the studies in the literature. It was thought that not examining the depth of tumor invasion with enough samples may affect the survival.
In a systematic review by Søreide et al. [19] for pT1b tumors, it was reported that re-resection is necessary with a low level of evidence. Jang et al. [20] reported that open-laparoscopic surgical technique and simple cholecystectomy-extended cholecystectomy treatment approach for T1a and T1b tumors did not make a difference in terms of 5-year survival. Although discussions on the surgical methods applied to continue, there is a consensus on resection with a tumor-free surgical margin. It is the most important factor for long survival and curative treatment [21]. In our study, it was determined that radical surgical intervention preferences were at the forefront in tumors with a depth of invasion above T1a.
Lymph node metastasis rates in pT1b tumors are reported to be 13% [22]. While the 5-year survival rate is 80%–100% in these patients without lymphatic metastases, it regresses to 8% in the presence of lymphatic metastases [19]. Segment 4b and 5 resection and regional lymphadenectomy were performed in 10 of 11 patients with T1b. N1 node involvement was detected in 1 of these patients after lymph node dissection. One patient was excluded from the survey analysis because he did not accept a second surgical intervention.
In pT2 tumors, surgical treatment is recommended with a tumor-free surgical margin, usually, segment 4b and 5 liver resection and regional lymph node resection. The surgical treatment recommended for pT3 tumors is similar. While lymph node enlargement was 27%–33% in pT2s, this rate was reported as 59% in pT3 tumors [22]. Survival in T3 tumors is very poor, about 8%–30% [18]. Surgery is recommended for all pT2 and pT3 patients in good condition to undergo major abdominal surgery and does not have inoperable findings [23]. Nine patients with pT2 and four patients with pT3 underwent lymphadenectomy with segmental or major liver resection. Lymph node involvement was detected in 3 patients with pT2 and 3 patients with pT3. It was observed that lymph node involvement caused stage progression and adversely affected survival in Kaplan-Meier analysis.
Although survival rates were similar in studies comparing hepatic resection and segmental hepatic resection for pT2 and pT3 tumors, complication rates were reported to be twice as high in patients who underwent hepatic resection [24]. Araida et al. [25] reported the complication rate as 10% in patients who underwent segmental resection. In our study, complications developed in 1 of the 2 patients who underwent hepatic resection and 4 of 20 patients who underwent segmental resection. The complication rate was similar to the rates reported in the literature. Most of the complications were at a low stage according to the Clavien-Dindo classification.
The limitations of our study were the unknown lymph node status of T1a patients, and the inability to evaluate the adequacy of the samples taken in the first histopathological examination of the patients.
In conclusion, the increasing use of laparoscopic cholecystectomy over the past 20 years has led to a notable increase in the number of incidental GBCs. Appropriate staging and histopathological evaluation are essential in guiding the surgeon’s operation. Histopathological evaluation is important in determining the T stage, which is the most effective parameter on patient survival and residual recurrences. Particular attention should be paid to the distinction between pT1a and pT1b. Surgery is the most important part of GBC management and the only potentially curative method.
References
1. Fuks D, Regimbeau JM, Le Treut YP, Bachellier P, Raventos A, Pruvot FR, et al. Incidental gallbladder cancer by the AFC-GBC-2009 Study Group. World J Surg. 2011; 35:1887–1897. PMID: 21547420.

2. Pitt SC, Jin LX, Hall BL, Strasberg SM, Pitt HA. Incidental gallbladder cancer at cholecystectomy: when should the surgeon be suspicious? Ann Surg. 2014; 260:128–133. PMID: 24509205.
3. Ahn Y, Park CS, Hwang S, Jang HJ, Choi KM, Lee SG. Incidental gallbladder cancer after routine cholecystectomy: when should we suspect it preoperatively and what are predictors of patient survival? Ann Surg Treat Res. 2016; 90:131–138. PMID: 26942156.

4. Nagarajan G, Kundalia K. Should every cholecystectomy specimen be sent for histopathology to identify incidental gall bladder cancer? Indian J Cancer. 2020; 57:2–3. PMID: 32129293.

5. Zaidi MY, Abou-Alfa GK, Ethun CG, Shrikhande SV, Goel M, Nervi B, et al. Evaluation and management of incidental gallbladder cancer. Chin Clin Oncol. 2019; 8:37. PMID: 31431030.

6. Chan KM, Yu MC, Lee WC, Jan YY, Chen MF. Adenosquamous/squamous cell carcinoma of the gallbladder. J Surg Oncol. 2007; 95:129–134. PMID: 17262729.

7. Agarwal AK, Kalayarasan R, Singh S, Javed A, Sakhuja P. All cholecystectomy specimens must be sent for histopathology to detect inapparent gallbladder cancer. HPB (Oxford). 2012; 14:269–273. PMID: 22404266.

8. Fahim RB, McDonald JR, Richards JC, Ferris DO. Carcinoma of the gallbladder: a study of its modes of spread. Ann Surg. 1962; 156:114–124. PMID: 13891308.
9. Pawlik TM, Gleisner AL, Vigano L, Kooby DA, Bauer TW, Frilling A, et al. Incidence of finding residual disease for incidental gallbladder carcinoma: implications for re-resection. J Gastrointest Surg. 2007; 11:1478–1487. PMID: 17846848.

10. Butte JM, Kingham TP, Gönen M, D’Angelica MI, Allen PJ, Fong Y, et al. Residual disease predicts outcomes after definitive resection for incidental gallbladder cancer. J Am Coll Surg. 2014; 219:416–429. PMID: 25087941.

11. Fong Y, Malhotra S. Gallbladder cancer: recent advances and current guidelines for surgical therapy. Adv Surg. 2001; 35:1–20. PMID: 11579805.
12. Lundgren L, Muszynska C, Ros A, Persson G, Gimm O, Valter L, et al. Are incidental gallbladder cancers missed with a selective approach of gallbladder histology at cholecystectomy? World J Surg. 2018; 42:1092–1099. PMID: 28900706.

13. Chatelain D, Fuks D, Farges O, Attencourt C, Pruvot FR, Regimbeau JM. Pathology report assessment of incidental gallbladder carcinoma diagnosed from cholecystectomy specimens: results of a French multicentre survey. Dig Liver Dis. 2013; 45:1056–1060. PMID: 23948233.

14. Ethun CG, Postlewait LM, Le N, Pawlik TM, Buettner S, Poultsides G, et al. A novel pathology-based preoperative risk score to predict locoregional residual and distant disease and survival for incidental gallbladder cancer: a 10-institution study from the U.S. Extrahepatic Biliary Malignancy Consortium. Ann Surg Oncol. 2017; 24:1343–1350. PMID: 27812827.

15. Geramizadeh B, Kashkooe A. Incidental gall bladder adenocarcinoma in cholecystectomy specimens; a single center experience and review of the literature. Middle East J Dig Dis. 2018; 10:249–253. PMID: 31049173.

16. Muszynska C, Lundgren L, Lindell G, Andersson R, Nilsson J, Sandström P, et al. Predictors of incidental gallbladder cancer in patients undergoing cholecystectomy for benign gallbladder disease: results from a population-based gallstone surgery registry. Surgery. 2017; 162:256–263. PMID: 28400123.

17. Hamdani NH, Qadri SK, Aggarwalla R, Bhartia VK, Chaudhuri S, Debakshi S, et al. Clinicopathological study of gall bladder carcinoma with special reference to gallstones: our 8-year experience from eastern India. Asian Pac J Cancer Prev. 2012; 13:5613–5617. PMID: 23317226.

18. Kwon AH, Imamura A, Kitade H, Kamiyama Y. Unsuspected gallbladder cancer diagnosed during or after laparoscopic cholecystectomy. J Surg Oncol. 2008; 97:241–245. PMID: 18095299.

19. Søreide K, Guest RV, Harrison EM, Kendall TJ, Garden OJ, Wigmore SJ. Systematic review of management of incidental gallbladder cancer after cholecystectomy. Br J Surg. 2019; 106:32–45. PMID: 30582640.

20. Jang JY, Heo JS, Han Y, Chang J, Kim JR, Kim H, et al. Impact of type of surgery on survival outcome in patients with early gallbladder cancer in the era of minimally invasive surgery: oncologic safety of laparoscopic surgery. Medicine (Baltimore). 2016; 95:e3675. PMID: 27258495.
21. Aloia TA, Járufe N, Javle M, Maithel SK, Roa JC, Adsay V, et al. Gallbladder cancer: expert consensus statement. HPB (Oxford). 2015; 17:681–690. PMID: 26172135.

22. Krell RW, Wei AC. Gallbladder cancer: surgical management. Chin Clin Oncol. 2019; 8:36. PMID: 31431029.

23. Benson AB, D’Angelica MI, Abbott DE, Abrams TA, Alberts SR, Anaya DA, et al. Guidelines insights: hepatobiliary cancers, version 2.2019. J Natl Compr Canc Netw. 2019; 17:302–310. PMID: 30959462.

24. D’Angelica M, Dalal KM, DeMatteo RP, Fong Y, Blumgart LH, Jarnagin WR. Analysis of the extent of resection for adenocarcinoma of the gallbladder. Ann Surg Oncol. 2009; 16:806–816. PMID: 18985272.

25. Araida T, Higuchi R, Hamano M, Kodera Y, Takeshita N, Ota T, et al. Hepatic resection in 485 R0 pT2 and pT3 cases of advanced carcinoma of the gallbladder: results of a Japanese Society of Biliary Surgery survey: a multicenter study. J Hepatobiliary Pancreat Surg. 2009; 16:204–215. PMID: 19219399.

Fig. 1
(A) Hepatoduodenal ligament dissection. (B) Diaphragmatic facial tumor implants (arrows). (C) Determination of hepatic resection line. (D) Hepatic parenchyma resection. Ch, choledochus; HA, hepatic artery; PV, portal vein.
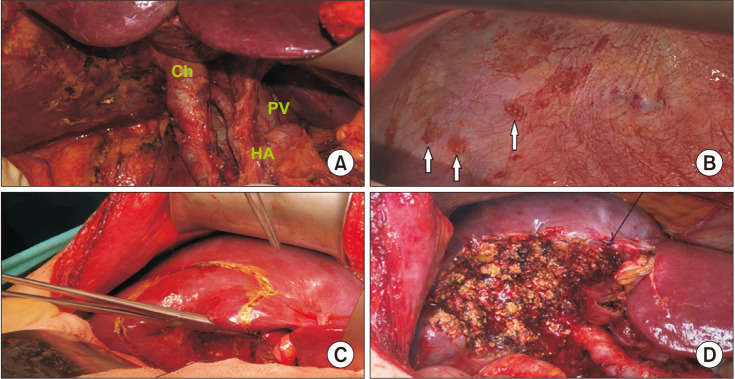
Fig. 2
Kaplan-Meier survival curves by the depth of invasion (pathologic [p] T) in all incidental gallbladder cancer (IGBC) patients (n = 40). pT1a, tumor invades the lamina propria; pT1b, tumor invades the muscular layer; pT2, tumor invades the perimuscular connective tissue on the peritoneal side, without the involvement of the serosa or tumor invades the perimuscular connective tissue on the hepatic side, with no extension into the liver; pT3, tumor perforates the serosa (visceral peritoneum) and/or directly invades the liver and/or 1 other adjacent organ or structure.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download