|
rs11574129 |
273 (72.8%)/98 (26.1%)/4 (1.1%) |
89 (68.5%)/36 (27.7%)/5 (3.8%) |
1.27 (0.85–1.91) |
0.240 |
1.18 (0.75–1.86) |
0.470 |
3.64 (0.91–14.56) |
0.070 |
|
rs3847987 |
254 (67.4%)/116 (30.8%)/7 (1.9%) |
81 (62.3%)/43 (33.1%)/6 (4.6%) |
1.25 (0.86–1.82) |
0.250 |
1.21 (0.78–1.86) |
0.390 |
2.17 (0.69–6.86) |
0.200 |
|
rs739837 |
226 (60.1%)/134 (35.6%)/16 (4.3%) |
69 (53.5%)/50 (38.8%)/10 (7.8%) |
1.24 (0.88–1.73) |
0.220 |
1.26 (0.83–1.90) |
0.290 |
1.50 (0.64–3.49) |
0.350 |
|
rs731236 |
344 (91.2%)/30 (8%)/3 (0.8%) |
116 (89.2%)/13 (10%)/1 (0.8%) |
1.08 (0.59–1.96) |
0.810 |
1.15 (0.58–2.27) |
0.690 |
0.62 (0.06–6.39) |
0.680 |
|
rs7975232 |
227 (60.2%)/134 (35.5%)/16 (4.2%) |
69 (53.1%)/51 (39.2%)/10 (7.7%) |
1.25 (0.89–1.75) |
0.200 |
1.28 (0.84–1.93) |
0.250 |
1.49 (0.64–3.47) |
0.360 |
|
rs757343 |
255 (67.6%)/115 (30.5%)/7 (1.9%) |
81 (62.3%)/43 (33.1%)/6 (4.6%) |
1.26 (0.87–1.84) |
0.230 |
1.22 (0.79–1.88) |
0.370 |
2.17 (0.69–6.86) |
0.200 |
|
rs1544410 |
348 (92.3%)/26 (6.9%)/3 (0.8%) |
118 (90.8%)/11 (8.5%)/1 (0.8%) |
1.06 (0.57–2.00) |
0.850 |
1.14 (0.55–2.35) |
0.720 |
0.62 (0.06–6.39) |
0.680 |
|
rs7963776 |
228 (60.8%)/131 (34.9%)/16 (4.3%) |
69 (53.9%)/50 (39.1%)/9 (7%) |
1.23 (0.88–1.73) |
0.230 |
1.27 (0.84–1.93) |
0.260 |
1.39 (0.58–3.31) |
0.460 |
|
rs2239185 |
229 (60.7%)/131 (34.8%)/17 (4.5%) |
68 (52.7%)/52 (40.3%)/9 (7%) |
1.24 (0.89–1.74) |
0.210 |
1.31 (0.86–1.98) |
0.210 |
1.31 (0.56–3.09) |
0.540 |
|
rs7305032 |
236 (62.8%)/123 (32.7%)/17 (4.5%) |
71 (55%)/50 (38.8%)/8 (6.2%) |
1.20 (0.85–1.68) |
0.300 |
1.32 (0.86–2.01) |
0.200 |
1.00 (0.41–2.45) |
1.000 |
|
rs2248098 |
217 (57.7%)/139 (37%)/20 (5.3%) |
67 (51.5%)/55 (42.3%)/8 (6.2%) |
1.11 (0.80–1.56) |
0.530 |
1.19 (0.78–1.80) |
0.410 |
0.96 (0.40–2.29) |
0.920 |
|
rs987849 |
213 (56.8%)/142 (37.9%)/20 (5.3%) |
64 (50%)/55 (43%)/9 (7%) |
1.14 (0.81–1.60) |
0.450 |
1.22 (0.81–1.86) |
0.340 |
1.00 (0.43–2.32) |
0.990 |
|
rs2239182 |
224 (59.9%)/132 (35.3%)/18 (4.8%) |
74 (56.9%)/50 (38.5%)/6 (4.6%) |
1.05 (0.74–1.48) |
0.800 |
1.09 (0.72–1.65) |
0.700 |
0.91 (0.34–2.41) |
0.850 |
|
rs2107301 |
193 (51.3%)/153 (40.7%)/30 (8%) |
66 (51.2%)/56 (43.4%)/7 (5.4%) |
0.91 (0.65–1.27) |
0.590 |
0.99 (0.65–1.50) |
0.950 |
0.59 (0.25–1.42) |
0.220 |
|
rs2239181 |
236 (62.6%)/128 (34%)/13 (3.5%) |
82 (63.1%)/38 (29.2%)/10 (7.7%) |
1.03 (0.72–1.47) |
0.870 |
0.88 (0.57–1.35) |
0.570 |
2.14 (0.89–5.14) |
0.095 |
|
rs2239180 |
235 (62.5%)/128 (34%)/13 (3.5%) |
81 (62.8%)/38 (29.5%)/10 (7.8%) |
1.04 (0.73–1.48) |
0.840 |
0.89 (0.58–1.37) |
0.600 |
2.15 (0.90–5.16) |
0.093 |
|
rs2238139 |
342 (91%)/33 (8.8%)/1 (0.3%) |
119 (91.5%)/11 (8.5%)/0 (0%) |
0.83 (0.40–1.70) |
0.600 |
0.83 (0.40–1.74) |
0.630 |
0.00 (0.00–NA) |
0.610 |
|
rs2239179 |
226 (60%)/134 (35.5%)/17 (4.5%) |
77 (59.7%)/48 (37.2%)/4 (3.1%) |
0.96 (0.67–1.38) |
0.820 |
1.02 (0.67–1.55) |
0.930 |
0.61 (0.20–1.92) |
0.380 |
|
rs12717991 |
200 (53%)/150 (39.8%)/27 (7.2%) |
70 (53.9%)/53 (40.8%)/7 (5.4%) |
0.90 (0.65–1.26) |
0.550 |
0.95 (0.63–1.43) |
0.800 |
0.65 (0.27–1.58) |
0.330 |
|
rs886441 |
342 (90.7%)/34 (9%)/1 (0.3%) |
119 (91.5%)/11 (8.5%)/0 (0%) |
0.80 (0.39–1.65) |
0.540 |
0.81 (0.39–1.68) |
0.560 |
0.00 (0.00–NA) |
0.610 |
|
rs2189480 |
156 (41.4%)/180 (47.8%)/41 (10.9%) |
54 (41.9%)/64 (49.6%)/11 (8.5%) |
0.90 (0.65–1.24) |
0.510 |
0.95 (0.62–1.44) |
0.800 |
0.69 (0.34–1.43) |
0.310 |
|
rs3819545 |
204 (54.3%)/144 (38.3%)/28 (7.5%) |
68 (52.7%)/55 (42.6%)/6 (4.7%) |
0.93 (0.67–1.30) |
0.690 |
1.05 (0.69–1.59) |
0.820 |
0.52 (0.20–1.31) |
0.140 |
|
rs3782905 |
284 (75.3%)/83 (22%)/10 (2.6%) |
98 (75.4%)/30 (23.1%)/2 (1.5%) |
0.98 (0.65–1.49) |
0.920 |
1.05 (0.65–1.70) |
0.840 |
0.52 (0.11–2.45) |
0.380 |
|
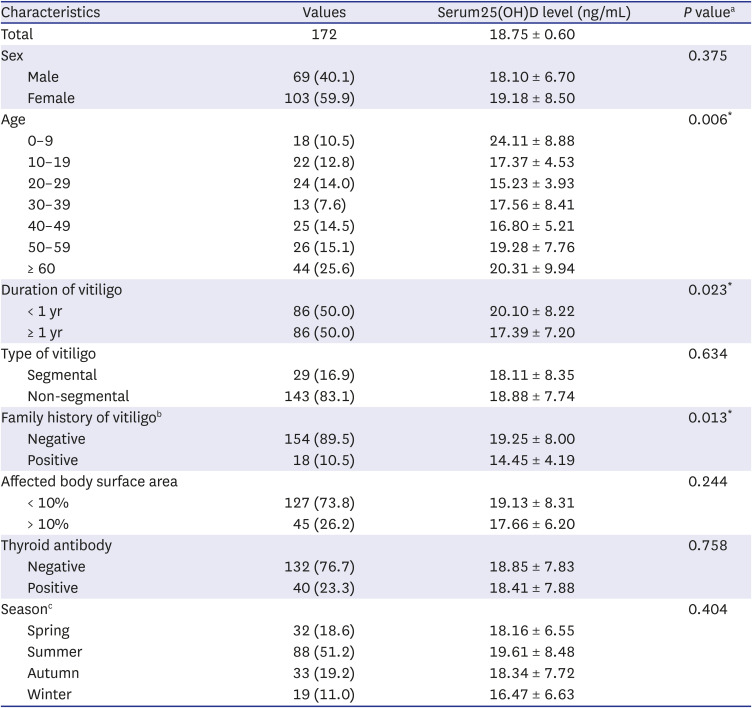
rs2239186 |
97 (25.8%)/193 (51.3%)/86 (22.9%) |
21 (16.8%)/77 (61.6%)/27 (21.6%) |
1.29 (0.94–1.77) |
0.110 |
1.89 (1.10–3.23) |
0.017a
|
1.05 (0.63–1.75) |
0.840 |
|
rs11168275 |
144 (38.3%)/168 (44.7%)/64 (17%) |
54 (41.5%)/61 (46.9%)/15 (11.5%) |
0.83 (0.62–1.13) |
0.230 |
0.88 (0.58–1.34) |
0.540 |
0.63 (0.34–1.17) |
0.130 |
|
rs2408876 |
150 (40.1%)/187 (50%)/37 (9.9%) |
53 (42.7%)/55 (44.4%)/16 (12.9%) |
1.00 (0.72–1.37) |
0.980 |
0.91 (0.60–1.40) |
0.680 |
1.22 (0.64–2.32) |
0.560 |
|
rs2238136 |
232 (61.5%)/134 (35.5%)/11 (2.9%) |
79 (60.8%)/45 (34.6%)/6 (4.6%) |
1.01 (0.70–1.46) |
0.950 |
0.97 (0.63–1.48) |
0.880 |
1.37 (0.46–4.02) |
0.580 |
|
rs2238135 |
198 (52.5%)/159 (42.2%)/20 (5.3%) |
61 (47.7%)/58 (45.3%)/9 (7%) |
1.12 (0.79–1.57) |
0.520 |
1.12 (0.74–1.70) |
0.590 |
1.24 (0.53–2.90) |
0.620 |
|
rs2853564 |
186 (49.3%)/151 (40%)/40 (10.6%) |
57 (44.2%)/57 (44.2%)/15 (11.6%) |
1.22 (0.90–1.66) |
0.200 |
1.29 (0.85–1.95) |
0.230 |
1.32 (0.68–2.53) |
0.420 |
|
rs4760648 |
119 (31.6%)/170 (45.1%)/88 (23.3%) |
45 (35.7%)/64 (50.8%)/17 (13.5%) |
0.75 (0.56–1.01) |
0.058 |
0.79 (0.51–1.22) |
0.290 |
0.54 (0.30–0.96) |
0.029a
|
|
rs10875694 |
266 (70.7%)/106 (28.2%)/4 (1.1%) |
85 (65.9%)/41 (31.8%)/3 (2.3%) |
1.17 (0.78–1.76) |
0.460 |
1.17 (0.75–1.83) |
0.490 |
1.36 (0.27–6.74) |
0.710 |
|
rs11168287 |
172 (45.6%)/168 (44.6%)/37 (9.8%) |
67 (51.5%)/51 (39.2%)/12 (9.2%) |
0.95 (0.69–1.30) |
0.730 |
0.90 (0.59–1.36) |
0.620 |
1.03 (0.51–2.08) |
0.930 |
|
rs4237855 |
116 (30.8%)/190 (50.4%)/71 (18.8%) |
58 (47.1%)/49 (39.8%)/16 (13%) |
0.67 (0.49–0.92) |
0.012a
|
0.56 (0.36–0.86) |
0.009a
|
0.67 (0.37–1.22) |
0.180 |
|
rs7302235 |
133 (35.6%)/181 (48.4%)/60 (16%) |
62 (48.1%)/48 (37.2%)/19 (14.7%) |
0.82 (0.61–1.11) |
0.200 |
0.68 (0.44–1.04) |
0.075 |
0.98 (0.55–1.74) |
0.940 |
|
rs4760655 |
123 (32.6%)/194 (51.5%)/60 (15.9%) |
39 (30%)/55 (42.3%)/36 (27.7%) |
1.24 (0.93–1.67) |
0.150 |
1.07 (0.68–1.68) |
0.760 |
1.75 (1.06–2.87) |
0.030 |
|
rs12581281 |
280 (74.5%)/88 (23.4%)/8 (2.1%) |
99 (76.7%)/28 (21.7%)/2 (1.6%) |
0.88 (0.57–1.36) |
0.560 |
0.90 (0.55–1.46) |
0.660 |
0.60 (0.12–3.00) |
0.520 |
|
rs7299460 |
113 (30%)/201 (53.3%)/63 (16.7%) |
33 (25.8%)/59 (46.1%)/36 (28.1%) |
1.29 (0.95–1.74) |
0.100 |
1.17 (0.73–1.87) |
0.500 |
1.69 (1.03–2.76) |
0.041a
|




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download