This article has been
cited by other articles in ScienceCentral.
Abstract
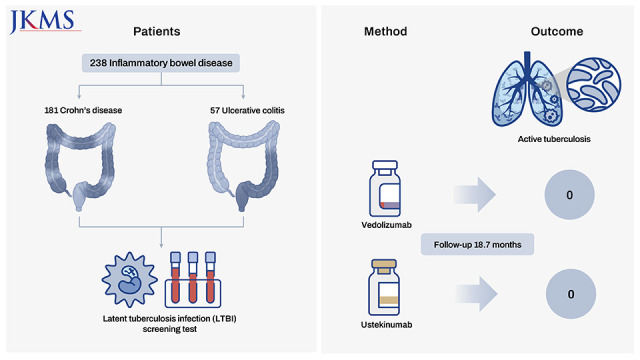
The present study investigated the risk of active tuberculosis in patients with inflammatory bowel disease (IBD) treated with vedolizumab or ustekinumab, in actual clinical settings in a country with an intermediate tuberculosis burden. The medical records of 238 patients with IBD who received vedolizumab or ustekinumab were retrospectively reviewed at a tertiary referral center in South Korea. All patients had ≥ 3 months of follow-up duration and underwent a latent tuberculosis infection screening test before initiation of the administration of these drugs. Of the 238 patients enrolled, 181 had Crohn’s disease, and 57 had ulcerative colitis. During the median 18.7 months of follow-up, active tuberculosis did not develop in any patient treated with vedolizumab or ustekinumab. Therefore, we concluded that the risk of tuberculosis appears to be low in patients with IBD treated with vedolizumab or ustekinumab in South Korea.
Go to :

Graphical Abstract
Go to :

Keywords: Vedolizumab, Ustekinumab, Tuberculosis, Inflammatory Bowel Disease
Despite the established effect of tumor necrosis factor (TNF) inhibitors in treating inflammatory bowel disease (IBD), patients who receive TNF inhibitors have a higher risk of developing active tuberculosis, because TNF plays an important role in the formation and maintenance of the integrity of the granuloma.
1 Vedolizumab (a monoclonal antibody that inhibits the α
4β
7 integrin heterodimer) and ustekinumab (a monoclonal antibody targeting the p40 subunit of interleukin-12 and interleukin-23) are novel agents for treating IBD.
2 Since the drug target of vedolizumab is not related to TNF, and the molecular mechanism of action of ustekinumab is indirectly associated with TNF inhibition,
3 the associated risk of tuberculosis with these two drugs is insignificant compared to TNF inhibitors, as reported in previous studies.
45678 However, all these studies were clinical trials, and they do not reflect real-world clinical experience. Therefore, we aimed to determine the risk of active tuberculosis in patients with IBD treated with vedolizumab or ustekinumab, in South Korea, a country burdened with tuberculosis at an intermediate level (the incidence was 59 per 100,000 person-years in 2019).
9
Patients were selected from the Asan Medical Center, a 2,700-bed referral hospital in Seoul, South Korea. Since the treatment with vedolizumab or ustekinumab was initiated in 2017 at our center, we first enrolled the patients with IBD who received vedolizumab or ustekinumab at least once from January 2017 to December 2020; we identified a total of 251 patients. After excluding those who did not undergo a latent tuberculosis infection (LTBI) screening test (n = 11) and for whom follow-up duration was <3 months after the initiation of vedolizumab or ustekinumab (n = 2), we retrospectively reviewed the medical records of the remaining 238 patients in June 2021. For LTBI screening, a tuberculin skin test and interferon-gamma release assay (QuantiFERON-TB Gold In-Tube or QuantiFERON-TB plus) were conducted on the same day,
10 along with a medical interview regarding tuberculosis and a chest radiograph. The detailed screening strategy, interpretation, and criteria for LTBI treatment at our center were previously described.
11
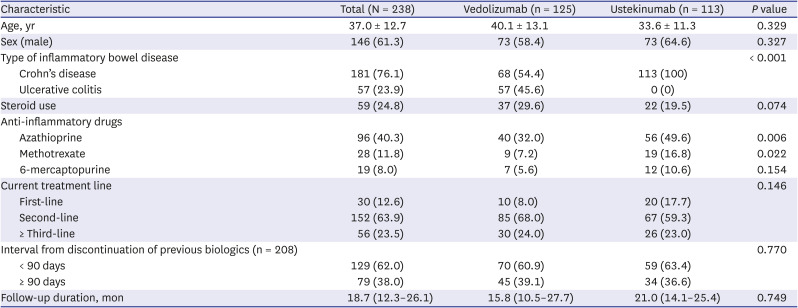
Of the 238 patients enrolled, 181 had Crohn’s disease and 57 had ulcerative colitis. The mean age of the 238 patients was 37.0 ± 12.7 years, and 61.3% were men. A total of 125 patients received vedolizumab, whereas the remaining 113 were treated with ustekinumab. Statistically significant differences in several clinical characteristics were noted between the vedolizumab and ustekinumab groups, including the type of IBD, steroid use, and the number of patients treated with an anti-inflammatory drug (
Table 1). Vedolizumab or ustekinumab was used as a first-line biologic in 12.6% (30/238) of the patients and as a ≥ second-line drug in the remaining 208 (87.4%). Of these 208 patients, the interval between the end of previous biologics and the initiation of vedolizumab or ustekinumab was < 90 days in 129 (62.0%). The LTBI screening test was performed in all 238 patients, of which, 29 (12.2%) had a positive result. Among these 29 patients, 12 patients successfully completed LTBI treatment. The remaining 17 patients did not receive LTBI treatment due to an adequate treatment history of LTBI or active tuberculosis.
Table 1
Baseline characteristics of the 238 patients with inflammatory bowel disease treated with vedolizumab or ustekinumab
|
Characteristic |
Total (N = 238) |
Vedolizumab (n = 125) |
Ustekinumab (n = 113) |
P value |
|
Age, yr |
37.0 ± 12.7 |
40.1 ± 13.1 |
33.6 ± 11.3 |
0.329 |
|
Sex (male) |
146 (61.3) |
73 (58.4) |
73 (64.6) |
0.327 |
|
Type of inflammatory bowel disease |
|
|
|
< 0.001 |
|
Crohn’s disease |
181 (76.1) |
68 (54.4) |
113 (100) |
|
Ulcerative colitis |
57 (23.9) |
57 (45.6) |
0 (0) |
|
Steroid use |
59 (24.8) |
37 (29.6) |
22 (19.5) |
0.074 |
|
Anti-inflammatory drugs |
|
|
|
|
|
Azathioprine |
96 (40.3) |
40 (32.0) |
56 (49.6) |
0.006 |
|
Methotrexate |
28 (11.8) |
9 (7.2) |
19 (16.8) |
0.022 |
|
6-mercaptopurine |
19 (8.0) |
7 (5.6) |
12 (10.6) |
0.154 |
|
Current treatment line |
|
|
|
0.146 |
|
First-line |
30 (12.6) |
10 (8.0) |
20 (17.7) |
|
Second-line |
152 (63.9) |
85 (68.0) |
67 (59.3) |
|
≥ Third-line |
56 (23.5) |
30 (24.0) |
26 (23.0) |
|
Interval from discontinuation of previous biologics (n = 208) |
|
|
|
0.770 |
|
< 90 days |
129 (62.0) |
70 (60.9) |
59 (63.4) |
|
≥ 90 days |
79 (38.0) |
45 (39.1) |
34 (36.6) |
|
Follow-up duration, mon |
18.7 (12.3–26.1) |
15.8 (10.5–27.7) |
21.0 (14.1–25.4) |
0.749 |

After commencement of treatment with vedolizumab or ustekinumab, the median follow-up was 18.7 months (interquartile range [IQR], 12.3–26.1), which was similar for both drugs (15.8; [10.5–27.7] vs. 21.0 [14.1–25.4],
P = 0.749, respectively). During this follow-up period of the 238 patients, active tuberculosis did not develop in patients treated with vedolizumab or ustekinumab (
Fig. 1).
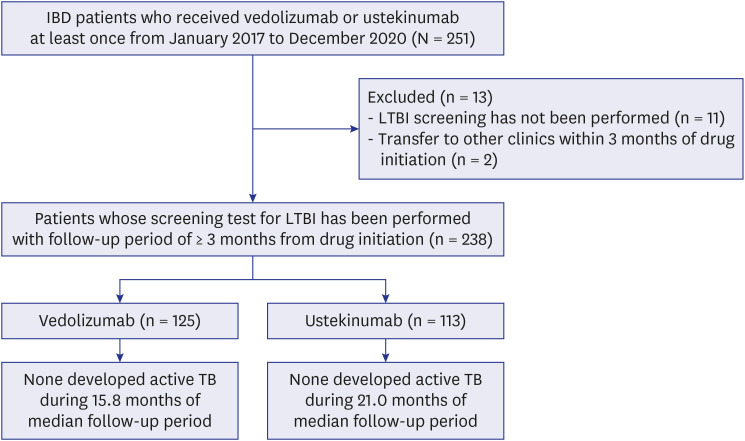 | Fig. 1
Flowchart of the study population.
IBD = inflammatory bowel disease, LTBI = latent tuberculosis infection, TB = tuberculosis.

|
This is the first study to investigate the risk of active tuberculosis in patients with IBD who were treated with vedolizumab or ustekinumab, in actual clinical settings at a tertiary referral center in South Korea. There is a risk of developing active tuberculosis after using TNF inhibitors in patients with IBD, which increases in proportion to the tuberculosis burden of each country.
12 Since South Korea is an intermediate tuberculosis burden country,
9 patients with IBD in South Korea who received TNF inhibitors have a high risk of developing tuberculosis. We have previously reported that, among patients with IBD treated with TNF inhibitors at our center from January 2011 to June 2017, the incidence of tuberculosis was 14 times higher than that of the general population.
11 Additionally, we recently reported that from 2001 to 2018, 1.46% (21/1,434) patients who received TNF inhibitors at our center developed active tuberculosis during the mean follow-up period of 49 months.
13 During the same period as this study (from 2017 to 2020), we also found that four (0.7%) patients were diagnosed with active tuberculosis among 582 patients treated with TNF inhibitors at least once. The median interval between the TNF inhibitor initiation and the diagnosis of active tuberculosis in these four patients was 7.0 months (IQR, 3.5–10.5). In contrast to this high risk of tuberculosis development after treatment with TNF inhibitors at our center, this study showed notable findings—none developed active tuberculosis among the 238 patients with IBD who were treated with vedolizumab or ustekinumab, during the median 18.7 months of follow-up.
It should be noted that we did not assert that the risk of tuberculosis due to vedolizumab or ustekinumab is negligible, based on our results. In contrast to the present study, several studies have reported the occurrence of tuberculosis after the use of vedolizumab or ustekinumab. A study reported three cases of pulmonary tuberculosis development among 3,677 patients with IBD in a clinical trial of vedolizumab, where the incidence corresponded to 0.1 per 100 patient-year.
14 Additionally, Sandborn et al.
15 reported the development of tuberculosis in one among 718 patients treated with ustekinumab. However, it is uncertain whether vedolizumab or ustekinumab should be considered as the causative agents for active tuberculosis in these studies, considering that immunosuppressive treatment other than TNF inhibitors (such as steroid or azathioprine) could also increase the risk of active tuberculosis.
16
Most patients in this study used vedolizumab or ustekinumab as a ≥ second-line agent, followed by TNF inhibitors. If active tuberculosis develops immediately after TNF inhibitor cessation, active tuberculosis that developed within three months of termination of TNF inhibitor was considered to have been caused by TNF inhibitors, in previous studies.
1718 Here, of the enrolled 208 patients, the interval between the end of the previous TNF inhibitor and the initiation of vedolizumab or ustekinumab was < 90 days in 129 patients (62.0%). Although none of the patients developed tuberculosis during this period, we believe that a real-world clinical study would present data concerning whether tuberculosis occurred during this transition period when the biologics change from TNF inhibitor to vedolizumab or ustekinumab.
Conclusively, we found that the risk of tuberculosis appears to be low in patients with IBD who were treated with vedolizumab or ustekinumab in South Korea. Considering that vedolizumab and ustekinumab showed similar therapeutic effects compared with TNF inhibitors,
1920 based on this study we suggest that these two novel agents could be regarded as optimal first-line choices for patients with IBD, in a country where the burden of tuberculosis is substantial, including South Korea.
Ethics statement
The study protocol was reviewed and approved by the Institutional Review Board of each center, including the Asan Medical Center (IRB No. 2021-0604). Informed consent was waived because of the retrospective nature of the study.
Go to :