Propensity score (PS) matching
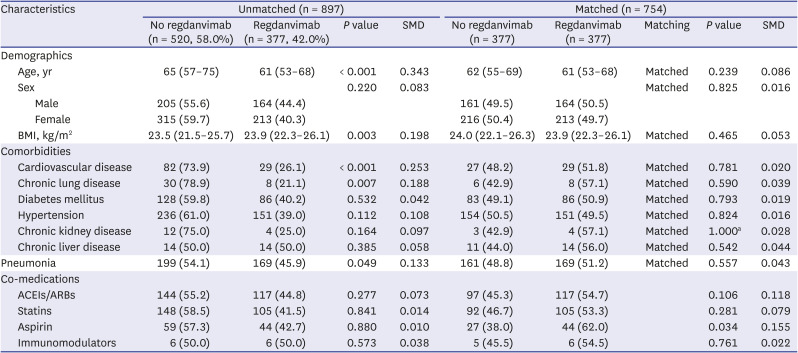
Factors collected in this study that were likely to affect treatment assignment or outcome were age, sex, body mass index (BMI), cardiovascular disease, chronic lung disease, diabetes mellitus, hypertension, chronic kidney disease, chronic liver diseases, pneumonia, angiotensin-converting enzyme inhibitors (ACEIs)/angiotensin receptor blockers (ARBs), statins, aspirin, and immunomodulators. Among them, to select variables to be included in the PS estimation model, we used both statistical method and clinical judgment, and gave priority to clinical judgment over statistical significance. As a statistical method, differences between groups were compared for each factor and treatment assignment or outcome, and it was judged to be significant if P < 0.05. As a result of the analysis, age, BMI, cardiovascular disease, chronic lung disease, and pneumonia were significantly different for treatment assignment, and age, cardiovascular disease, hypertension, chronic kidney disease, and pneumonia were significantly different for outcome. Factors that showed significant differences in both treatment assignment and outcome were age, cardiovascular disease, and pneumonia. In clinical judgment, all variables corresponding to the regdanvimab administration criteria, which were assumed to be related to both treatment assignment and outcome, were included, and this was extended to include not only Korean administration criteria at that time but also that of the European Medicines Agency (EMA). In addition, sex was also added based on the results of recent studies. Since covariates included in the PS estimation model should not be affected by treatment assignment and should be measured before assignment, the agents administered for the coronavirus disease 2019 (COVID-19) treatment after hospitalization were excluded.
Accordingly, the modeling of the PS estimation was performed using the following variable selection methods.
∘ All covariates collected (PS1): age, sex, BMI, cardiovascular disease, chronic lung disease, diabetes mellitus, hypertension, chronic kidney disease, chronic liver diseases, pneumonia, ACEIs/ARBs, statins, aspirin, immunomodulators
∘ Variables that were statistically significant for both treatment assignment and outcome, and significant for outcome (PS2): age, cardiovascular disease, hypertension, chronic kidney disease, pneumonia
∘ Variables corresponding to Korean regdanvimab administration criteria according to clinical judgment (PS3): age, cardiovascular disease, chronic lung disease, diabetes mellitus, hypertension, pneumonia
∘ Variables corresponding to Korean regdanvimab administration criteria according to clinical judgment, and variables that were statistically significant in the outcome (PS4): age, cardiovascular disease, chronic lung disease, diabetes mellitus, hypertension, chronic kidney disease, pneumonia
∘ Based on clinical judgment, the variables corresponding to EMA’s regdanvimab administration criteria, and sex (PS5): age, sex, BMI, cardiovascular disease, chronic lung disease, diabetes mellitus, hypertension, chronic kidney disease, chronic liver diseases, pneumonia
For each variable selection method, multivariable logistic regression analysis was performed to estimate the PS, and matching was performed using the calculated PS. To check the balance after matching, standardized mean difference (SMD) was used. If the absolute value of SMD > 0.1, it means that there is an imbalance of the covariate between the two groups.
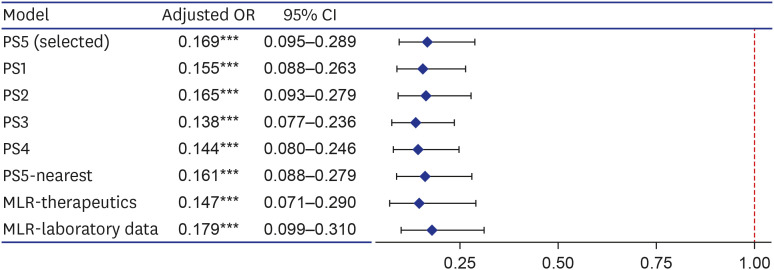
As a result of 1:1 optimal matching, the absolute SMD of age exceeded 0.1 only in PS1, and the absolute SMD values of all covariates were within 0.1 in other matching cohorts. So, we selected PS5, which is a balanced model for all covariates while including as many variables as possible according to clinical judgment, and then additionally performed 1:1 nearest matching with calipers. The caliper was designated as 0.2 times the standard deviation of the logit of the PS as it was known to be suitable for estimating the treatment effect. In the result of caliper-designated nearest matching, the absolute SMD values further decreased, but 25 patients in the treatment group were excluded and only 352 pairs of matching data were derived. In order to rule out the possibility that patients with a specific tendency were excluded, we selected the optimal matching result with sufficient balance without omission of patients in the treatment group as the final matching cohort. In addition, sensitivity analysis was performed for PS1-4 and PS5-nearest.
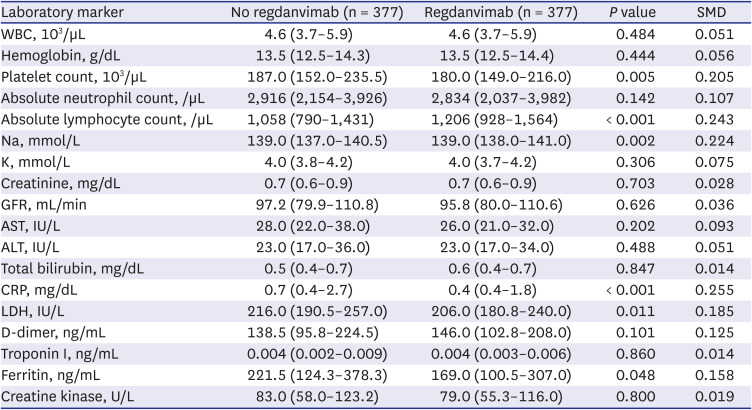
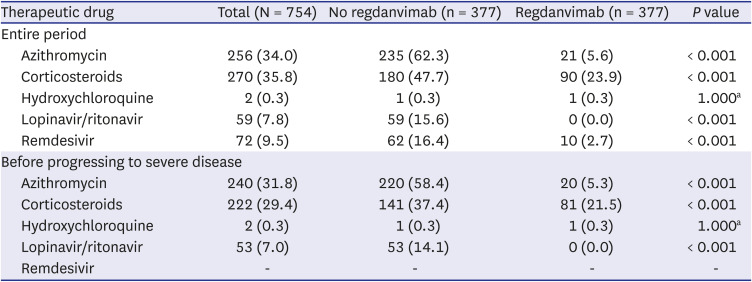
Among the baseline laboratory data, C-reactive protein, lactate dehydrogenase, D-dimer, troponin I, ferritin, and creatine kinase have not been clearly proven as prognostic factors for COVID-19, but have been reported to be significantly elevated in severe COVID-19 patients. Matching variables should have no missing values, and since many of the study subjects were missing these values, the laboratory data were excluded from the matching variables. However, these laboratory data may have influenced the outcome, so we added them as covariates in the sensitivity analysis. Similarly, azithromycin, corticosteroids, hydroxychloroquine, lopinavir/ritonavir, and remdesivir administered for the purpose of treating COVID-19 after hospitalization may have affected the outcome, so these were also added as covariates in the sensitivity analysis.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download