CASE DESCRIPTION
The patient was a 63-year-old woman (162 cm, 63.3 kg, O+), with no medical history including adverse reactions to vaccines. She received the 1st dose of the ChAdOx1 vaccine on June 19, 2021, and did not experience any side effects. She received a second dose of homologous inoculation on September 4, 2021, and developed chest pain as an initial symptom three hours after the second shot. Five days later, she visited the emergency department of a tertiary hospital for aggravated chest pain, dizziness, nausea, vomiting, diarrhea, and fever persisting for four consecutive days.
Echocardiography on the following day showed normal cardiac chamber dimensions with a mildly thick left ventricular (LV) wall (diastolic interventricular septum 12.6 mm and LV posterior wall 12.9 mm) with LV ejection fraction (EF) of 15% and insufficient transaortic blood flow by Doppler imaging. A trivial amount of pericardial effusion was also observed. Creatine kinase myocardial band (CK-MB), high-sensitive troponin I (hsTnI) and N-terminal pro-B type natriuretic peptide were as high as 91.3 ng/mL (normal range: 0.5–3.0 ng/mL), 29,808 pg/mL (normal range: 0–10 pg/mL), and 10,289 pg/mL (normal range: less than 450 pg/mL) respectively, and a coronary angiogram showed normal coronary arteries. Abdominal computed tomography revealed nonspecific findings and, vomiting, and diarrhea with fever did not persist. She had a blood pressure (BP) of 71/55 mmHg and a heart rate of 73 beats per min (bpm), which led to venoarterial (VA) extracorporeal membrane oxygenation (ECMO). As concurrent hemoptysis made her airway insecure, she was intubated and invasive mechanical ventilation was initiated. Her pulse waveform from the right radial artery became flat, and a temporary pacemaker (TPM) was needed for three days because of unstable electrical activity. Acute symptoms such as chest pain, dizziness, abnormal electrocardiogram (ECG), elevated cardiac biomarkers, and a thickened LV wall with severe systolic dysfunction facilitated the diagnosis of clinically suspected acute myocarditis according to previous diagnostic criteria.
25 Despite eight days of mechanical circulatory support, cardiac function did not improve, while pulmonary infiltration was newly detected in the right lower lung field on a chest radiograph. The patient was transferred to our institution for heart transplantation (HTx) without any delay on the same day. Under the VA-ECMO set at the blood flow of 3.4 L/min, the on-arrival vital signs were as follows; mean BP of 110 mmHg, heart rate of 58 bpm, respiratory rate of 21 breaths/min, and a body temperature of 36.2°C. We immediately performed an interatrial septostomy for LV decompression using a 25 mm balloon. Septostomy was performed via the left femoral vein, due to near-total occlusion of the right femoral vein, which was the access route used for the previous TPM. Rapidly progressing bilateral pulmonary infiltration was detected on a chest radiograph (
Fig. 1A) and the ventilator was set at a positive end-expiratory pressure of 5 cmH
2O with a FiO
2 of 100% and could not maintain a PaO
2 of 60 mmHg without ECMO. The CK-MB and hsTnI levels tested at our institution reached 7.5 ng/mL (normal range: 0.5–3.1) and 4,803 pg/mL (normal range: 0–11.6) respectively. Blood samples were negative for autoimmune markers and microbiological tests for pathogens, such as
Toxoplasma. A low voltage with a widened QRS was observed on the ECG (
Supplementary Fig. 1A). Echocardiography revealed an LVEF of 5%, absence of aortic valve opening, and ‘spontaneous echo contrast’ in the ascending aorta (
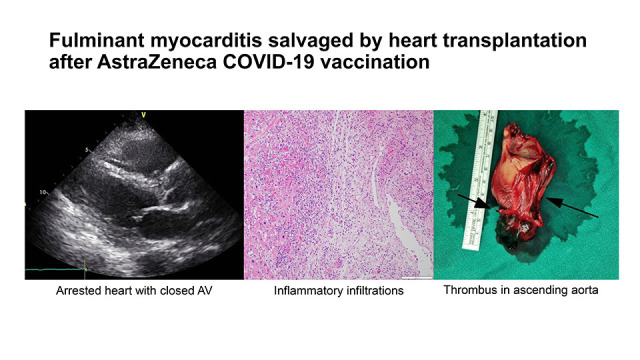
Fig. 2A). Our multidisciplinary cardiogenic shock team decided to list her for HTx because her chances of recovery seemed very low.
Fig. 1
Chest X-ray. (A) Bilateral pulmonary infiltration where right lung was developing. (B) Post-transplant X-ray turned into total whiteout.

Fig. 2
The evidences of thrombi. (A) Spontaneous echogenic material in the tubular portion of ascending aorta. (B) 7 cm-long huge thrombus of aortic root obtained intraoperatively. Thrombus in the form of left anterior descending artery (Long arrow), right coronary artery os (cut) (short arrow). The rest of linear thrombi occluding the right coronary artery is shown in the Fig. 2B. (C) Chest tomography displayed as a thrombus template.

On the fourth day after listing, a heart from a 17-year-old brain-dead male donor (171 cm, 90 kg, Rh O+) was allocated. One day before HTx surgery, her heart stopped, with a heart rate of zero on ECG (
Supplementary Fig. 1B). Her tidal volume also sharply declined from 400 to 180 mL within a few hours of the ECG change. We decided to proceed with HTx, expecting that lung congestion would improve.
The surgery was uneventful until the aortic cross-clamp was released. The total ischemic time was 157 minutes. Several large thrombi occluding the aortic root, right lower pulmonary vein, superior vena cava, and inferior vena cava were removed intraoperatively (
Fig. 2B and C). Biventricular contractility was normal with minimal support from the inotropes. However, upon initiation of weaning from cardiopulmonary bypass, the peripheral O
2 saturation decreased from 100% to 60% with massive sanguineous discharge from the endotracheal tube. Venovenous (VV) ECMO was used as a bridge-to-recovery strategy because of the congested lungs. After transportation from the operation room to the intensive care unit, the “flooding” discharge through the endotracheal tube required suction every 2–5 minutes for the next 9 hours. Bilateral white-out of the lungs was observed on a chest radiograph (
Fig. 1B). The pathological findings of the myocardium were consistent with myocarditis (
Fig. 3). The pathologic examination of the explanted heart revealed an inflammatory infiltration predominantly composed of T-cells and histiocytes that was noted in all four chambers of the heart. Although the specific etiology cannot be determined, these findings are consistent with acute lymphocytic myocarditis and similar to previous reports.
6
Fig. 3
Microscopic findings of the explanted heart. (A) Pathologic examination of the explanted heart revealed an inflammatory infiltration predominantly composed of T-cells and cardiomyocyte damage accompanied. (B) This inflammation was noted in the both atria and ventricles, and the interventricular septum. These findings were consistent with acute lymphocytic myocarditis. Furthermore, thrombi were noted in the lumen of the right coronary artery, and (C) nodal artery located adjacent the sinoatrial node. (D) The inflammatory cells were predominantly reactive for CD3, and many histiocytes were also noted.

As a cardiac index of 1.8–2.0 L/min/m2, and a mean pulmonary artery pressure of 30 mmHg were consistent with imminent right ventricular (RV) failure, we changed the ECMO configuration to veno-arteriovenous (VAV) (arterial system blood flow 3.5 L/min) settings to minimize blood flow to the RV.
After several adjustments, including nitric oxide inhalation and alveolar recruitment maneuvers, the patient’s vital signs stabilized. VAV-ECMO was then changed to VV-ECMO on a post-operative day 3. However, the bilateral damage to the lung parenchyma was more severe than expected, resulting in necrotizing pneumonia. The patient died on the 54th day from the beginning of the symptoms.
The presence of multiple thromboses (ascending aorta, coronary arteries, right lower pulmonary vein, right femoral vein, and superior and inferior vena cava) and initial thrombocytopenia (102,000 /µL) prompted testing of a postoperative blood sample for the anti-platelet factor 4 (anti-PF4) antibody, a marker of vaccine-induced thrombotic thrombocytopenia, by enzyme-linked immunosorbent assay.
7 However, this test was negative.
Ethics statement
This study was approved by the Institutional Review Board of the Pusan National University Yangsan Hospital (No. 2021-0553) and the requirement for informed consent was waived.
DISCUSSION
The incidence of myocarditis following SARS-CoV2 infection has been reported to be 150 cases per million in March 2020 and January 2021, which is approximately 16 times higher in subjects without COVID-19.
8 Given the high risk of myocarditis, the importance of vaccination may outweigh the risk of SARS-CoV-19 infection. The United States Centers for Disease Control and Prevention (US CDC) reported that males 12–29 years of age are associated with the highest risk of myocarditis, with 40.6 cases per million after the second dose of the mRNA COVID-19 vaccine.
9 A few cases associated with the ChAdOx1 nCoV-19 vaccine have also been reported.
10 There are limited data regarding the risk factors associated with myocarditis following adenoviral-vector vaccination. However, vaccine-related myocarditis was reported even before the COVID-19 era.
11
The US CDC and Brighton Collaboration define vaccine-related myocarditis as elevated cardiac biomarkers, abnormal ECG, abnormal echocardiographic/magnetic resonance findings consistent with myocarditis, and histologic confirmation.
1213 Previous pathological examination of the heart in myocarditis after COVID-19 mRNA vaccination showed multifocal cardiomyocyte damage associated with mixed inflammatory infiltration predominantly composed of T-cells and histiocytes, admixed with eosinophils, B cells and plasma cells in both ventricles.
6 However, the cause-and-effect relationship between the COVID-19 vaccine and myocarditis has not yet been established. Only the clinical context, in adjunction to the case definition, is available for deciding causal relationship. The Korea Disease Control and Prevention Agency adjudicates the causal relationship between the vaccine and adverse events based on certain factors, including clear evidence of vaccination, appropriate temporal association, the presence of widely recognized causality, and the absence of other possible causes.
14 Our patient’s symptoms, ECG, laboratory tests, echocardiography, and histologic findings met the criteria with a strong temporal association with the ChAdOx1 vaccine. However, this case was not confirmed as a definite COVID-19 vaccination-related myocarditis case from the KDCA. Nevertheless, physicians should always be aware of the possibility of vaccine-related myocarditis if there are no other identifiable causes of myocarditis with temporal relation of any kind of vaccination.
In addition, the European Medicines Agency and the Korean Ministry of Food and Drug Safety classified vaccine-induced thrombotic thrombocytopenia (VITT) as a serious adverse effect caused by COVID-19 vaccines using adenovirus vectors (such as the ChAdOx1 vaccine). Although our patient tested negative for anti-PF4 and other diagnoses, such as thrombus formation due to high LV afterload following VA-ECMO and disseminated intravascular coagulation, were feasible, the likelihood of VITT could not be confidently excluded as there were multiple thrombi present throughout the arterial and venous systems, suggesting that the criteria for probable VITT were met.
7
To the best of our knowledge, this is the first reported case of fulminant myocarditis following ChAdOx1 nCoV-19 vaccination salvaged by HTx. Vaccine-related fulminant myocarditis is rare but can be fatal. Clinicians need to be alert to the rapidly changing conditions of patients, regardless of the type of vaccine administered.







 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download