INTRODUCTION
Totally implantable venous access ports (TIVAPs) are widely used vascular access methods for total parenteral nutrition, fluids, blood products, administration of medical agents, chemotherapy, and hemodynamic monitoring.(
1) TIVAPs were introduced in the early 1980s and are currently the most common method for chemotherapy because of their various advantages in long-term administration.(
2) Most oncologic guidelines recommend implantable port placement if chemotherapy is required for more than 3 months.(
2-
4)
Traditionally, TIVAPs were inserted via the internal jugular vein and the injection port was mounted on the anterior chest wall. However, this method cannot be applied to all patients, specifically those with malignancies in the neck or a tracheostomy.(
5) In addition, many patients are uncomfortable about having a port inserted in the chest wall, especially breast cancer patients with large lesions.(
5,
6) Recently, there has been an increase in the use of implantable ports in the arm, especially in breast cancer patients, because of the simplicity of the procedure and cosmetic superiority.(
2,
6) However, few clinical trials have investigated the safety and stability of the procedure in various types of cancers. Other studies have reported that arm ports have a higher rate of complications than chest ports, especially with regard to catheter-induced thrombosis.(
7) Against this background, we aimed to determine the differences in the safety and clinical usefulness of ultrasound-guided placement of implantable ports in the arm compared to the chest when used in solid organ cancer patients.
Go to :

RESULTS
The total number of patients included in the study was 371, of which 119 had an implantable port in the chest and 252 had an implantable port in the arm. The mean age was 58.6 years, and there were more women in the arm port group. The types of cancer included were colon cancer (157 patients; 42.3%), breast cancer (156; 42.1%), gastric cancer (38; 10.2%), rectal cancer (18; 4.9%), and pancreatic cancer (2; 0.5%). The use of an arm port was most common in breast cancer patients, while chest ports were most commonly used in colon cancer patients (P < 0.001). A total of 142 patients with metastatic cancer and 290 patients who received adjuvant chemotherapy were included. Among them, the chest port was used more frequently for metastatic cancer (49.6% vs 32.9%; P = 0.003) and adjuvant chemotherapy (86.6% vs 74.2%; P = 0.011) than the arm port. The demographics of the patients included in the study are summarized and described in detail in
Table 1.
Table 1
Demographics and Baseline Characteristics
|
Patient characteristics |
Chest (n = 119) |
Arm (n = 252) |
Total (n = 371) |
P |
|
Age, year |
64.5 ± 11.4 |
55.8 ± 11.4 |
58.6 ± 12.1 |
<0.001 |
|
Female, n (%) |
52 (43.7) |
172 (68.3) |
224 (60.4) |
<0.001 |
|
Body mass index (kg/m2) |
23.2 ± 3.5 |
24.4 ± 3.9 |
24.0 ± 3.8 |
0.319 |
|
Weight (kg) |
59.5 ± 11.2 |
62.5 ± 11.0 |
61.6 ± 11.2 |
0.016 |
|
Height (cm) |
160.1 ± 8.6 |
160.1 ± 7.4 |
160.1 ± 7.8 |
0.964 |
|
Smoking |
13 (10.9) |
32 (12.7) |
45 (12.1) |
0.625 |
|
Hypertension |
49 (41.2) |
78 (31.0) |
127 (34.2) |
0.053 |
|
Diabetes mellitus |
19 (16.0) |
37 (14.7) |
56 (15.1) |
0.747 |
|
Cerebrovascular disease |
2 (1.7) |
6 (2.3) |
8 (2.1) |
0.122 |
|
Anti-Platelet agent |
6 (5.0) |
5 (2.0) |
11 (3.0) |
0.186 |
|
Anti-Coagulants |
0 (0.0) |
3 (1.2) |
3 (0.8) |
0.554 |
|
Cancer type |
|
|
|
<0.001 |
|
Breast |
15 (12.6) |
141 (56.0) |
156 (42.1) |
|
|
Colon |
88 (74.0) |
69 (27.4) |
157 (42.3) |
|
|
Rectum |
3 (2.5) |
15 (6.0) |
18 (4.9) |
|
|
Stomach |
13 (10.9) |
25 (9.9) |
38 (10.2) |
|
|
Pancreas |
0 (0.0) |
2 (0.8) |
2 (0.5) |
|
|
Metastasis |
59 (49.6) |
83 (32.9) |
142 (38.3) |
0.003 |
|
Adjuvant |
103 (86.6) |
187 (74.2) |
290 (78.2) |
0.011 |
|
Radiation therapy |
20 (16.8) |
128 (50.8) |
148 (39.9) |
<0.001 |

The success rate for both procedures was 100%. All patients using the chest port had a catheter inserted through the right or left internal jugular vein, and the ports were mounted on the anterior chest wall. The most common veins accessed in the arm port group were the basilic vein in 204 patients (81%), the cephalic vein in 35 patients (13.9%) and the brachial vein in 13 patients (5.1%). The mean length of the inserted conduit was 46.5, and the mean diameter of the veins was 3.1 mm (
Table 2).
Table 2
Detail Profile of Arm Port Procedures
Arm port
(n = 252) |
Right
(n = 74, 29.4%) |
Left
(n = 178, 70.6%) |
P |
|
Access vein, n (%) |
|
|
|
|
Basilic vein |
59 (79.7) |
145 (81.4) |
<0.001 |
|
Brachial vein |
1 (1.4) |
12 (6.8) |
0.006 |
|
Cephalic vein |
14 (18.9) |
21 (11.8) |
0.326 |
|
Length (cm) |
44.4 ± 3.2 |
47.5 ± 3.5 |
<0.001 |
|
Diameter (mm) |
3.2 ± 0.8 |
3.0 ± 0.7 |
0.120 |

The most common complications were infection-related insults (n = 28, 7.5%), and there was no statistically significant difference between the two groups in terms of events that could be deemed early complications, occurring within 2 weeks. There was no significant difference between the groups in terms of complications related to thrombosis (P = 0.139). Late complications (after 2 weeks), such as catheter-related blood stream infection (CRBSi), pocket infection, and wound dehiscence occurred more frequently in the chest port group than in the arm port group, and the difference was statistically significant (P = 0.031). Overall total complication rates were also lower in the arm port group than in the chest port group (P < 0.001). However, there was no difference in the duration of port implantation between the two groups (P = 0.456). The complication-free survival rate was higher in the arm port group than in the chest port group (P < 0.001,
Fig. 2). The chest port group had more cases of port removal due to complications than the arm port group (P = 0.002,
Table 3). In the univariate and multivariate comparative analyses for risk factors of complications, the use of a chest port was found to be independent risk factor with statistically significant (P = 0.002,
Table 4).
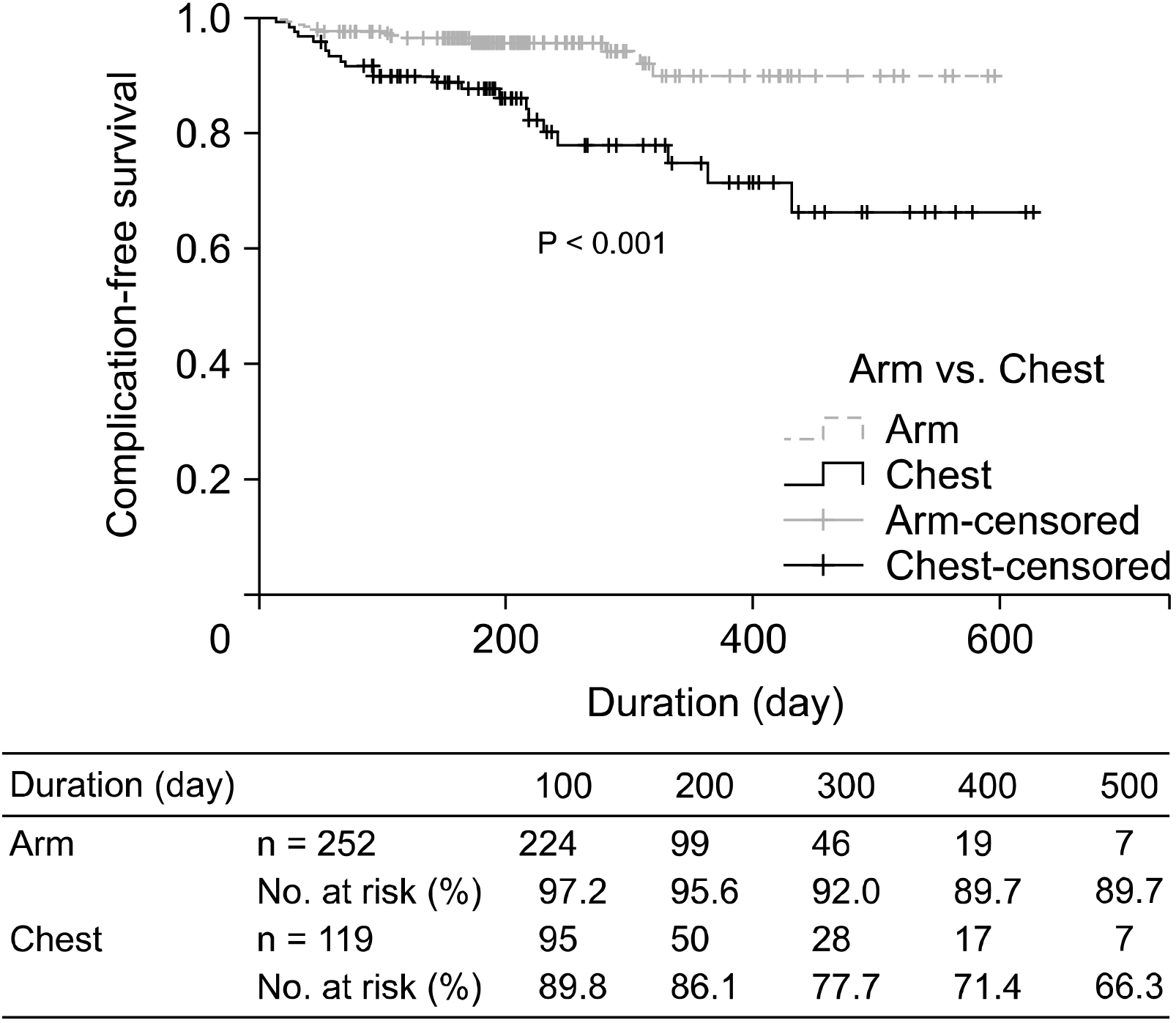 | Fig. 2Complication-free catheter survival. 
|
Table 3
Comparison of Port-Related Complication in Arm and Chest Ports
|
Follow-up |
Chest (n = 119) |
Arm (n = 252) |
Total (n = 371) |
P |
|
Thrombosis, n (%) |
5 (4.2) |
3 (1.2) |
8 (2.1) |
0.139 |
|
Early Infection, n (%) |
|
|
|
>0.999 |
|
CRBSI |
1 (50.0) |
1 (100) |
2 (66.7) |
|
|
Pocket infection |
1 (50.0) |
0 |
1 (33.3) |
|
|
Wound dehiscence |
0 |
0 |
0 |
|
|
Late Infection, n (%) |
|
|
|
0.031 |
|
CRBSI |
2 (12.5) |
6 (66.7) |
8 (32.0) |
|
|
Pocket infection |
8 (50.0) |
2 (22.2) |
10 (40.0) |
|
|
Wound dehiscence |
6 (37.5) |
1 (11.1) |
7 (28.0) |
|
|
No. of complication/1,000 catheter day |
23 (19.3) |
13 (5.2) |
36 (9.7) |
<0.001 |
|
Catheter day/port (day) |
225.1 ± 142.5 |
214.1 ± 109.8 |
217.6 ± 121.1 |
0.456 |
|
Port removal, n (%) |
|
|
|
0.002 |
|
Finish of CTx |
72 (60.5) |
155 (61.5) |
227 (61.2) |
|
|
Complication |
20 (16.8) |
12 (4.8) |
32 (8.6) |
|

Table 4
Univariate and Multivariate Analysis for Factors of Port-Related Complication
|
Factor (reference) |
Univariate |
|
Multivariate |
|
HR |
95% CI |
P |
HR |
95% CI |
P |
|
Sex (Female) |
1.265 |
0.650-2.462 |
0.488 |
|
1.243 |
0.590-2.617 |
0.567 |
|
Age (per year) |
0.989 |
0.962-1.017 |
0.453 |
|
0.969 |
0.940-0.998 |
0.040 |
|
BMI (per kg/m2) |
0.989 |
0.905-1.081 |
0.813 |
|
1.000 |
0.911-1.098 |
0.997 |
|
Location of port (Arm) |
0.297 |
0.149-0.591 |
0.005 |
|
0.240 |
0.115-0.503 |
0.002 |
|
Smoking |
0.861 |
0.304-2.442 |
0.778 |
|
0.986 |
0.324-3.003 |
0.981 |
|
Hypertension |
0.575 |
0.268-1.23 |
0.153 |
|
|
|
|
|
Diabetes mellitus |
0.802 |
0.310-2.075 |
0.649 |
|
|
|
|
|
Anti-Coagulants |
3.018 |
0.411-22.179 |
0.278 |
|
|
|
|
|
Cancer type (Breast) |
|
|
|
|
|
|
|
|
Colon and rectum |
1.265 |
0.613-2.612 |
0.525 |
|
|
|
|
|
Stomach |
0.720 |
0.201-2.579 |
0.614 |
|
|
|
|
|
Metastasis |
0.820 |
0.399-1.687 |
0.591 |
|
|
|
|
|
Neoadjuvant |
0.952 |
0.432-2.098 |
0.902 |
|
|
|
|
|
Radiation therapy |
0.766 |
0.365-1.608 |
0.481 |
|
|
|
|

Go to :

DISCUSSION
The use of TIVAPs is increasing because it can provide convenience and safety to patients who need frequent venous access.(
3,
7). Especially, its safety has been proven for cancer patients who need chemotherapy, and the use of TIVAPs is recommended in guidelines provided by many cancer-related societies.(
2,
3,
7,
8)
The most used vein for the TIVAPs procedure is the internal jugular vein, and a port is inserted in the upper portion of the anterior chest wall. However, patients who have undergone breast cancer surgery or have head and neck cancer tend to pose challenged for insertion or reluctant to use these traditional methods.(
2,
6,
7) In such situations, an arm port can be an alternative method. Compared to the chest port, the arm port has no difference in the incidence of complications or safety and is a preferred method for breast cancer patients who value cosmesis and have aesthetic considerations.(
5,
7)
The most important aspect of performing the arm port procedure is the accurate puncture of an appropriate vein without damaging the surrounding structures. As such, it is recommended to use the Seldinger technique under ultrasound guidance. As shown in a randomized controlled study, compared to the establishment of access through a cutdown procedure, the Seldinger technique is a much more effective and faster method for venous access and is highly recommended for most patients who require venous port access.(
9)
The location of the catheter tip is also an important factor in determining the outcome of the port function, and it is generally important to position the catheter tip at the confluence point of the right atrium and the superior vena cava. Therefore, radiologic confirmation must be achieved. (
5,
10,
11)
The position in which the port is inserted into the patient’s arm may also be an important factor in terms of patient convenience. If it is located close to the basilic vein, it is inconvenient because the patient needs to rotate the arm internally and the needling site may be disrupted, which may cause complications.(
7) In addition, if the basilic vein of the upper arm is punctured and the port is placed in the forearm, there may be limitations in arm movement, and the catheter may be damaged by continuous motion of the limb.(
7,
12) For this reason, upon puncture of the basilic or brachial veins of the upper arm in our hospital, the port is placed on the lateral side of the upper arm; meanwhile, when the cephalic vein is punctured, the port is inserted near the puncture site.
For veins of the upper arm to be punctured, it is generally recommended to use veins with a diameter of 3 mm or more.(
8,
10) We used basilic or cephalic veins with a diameter of more than 3 mm whenever possible; however, brachial veins were used when the diameter the other veins was less than 2 mm or if pathologies otherwise precluded the use of the superficial vein. In some cases, appropriate venous access was possible even with a diameter of 3 mm or less through careful, ultrasound-guidance. If vein access was still not possible, a cut-down procedure was performed through a 1 cm mini-incision, allowing for a technical success of 100%.
Although TIVAPs-related complications vary between several studies, they are reported at 0.3-1.8/1000 catheter days, and the most common complications are thrombosis and infection.(
6) A systemic review and retrospective study have shown that TIVAPs placements between the chest and the arm did not have significant difference in terms of adverse events for breast cancer patients. Moreover, patient satisfaction for arm port placement have shown better results than for chest port placement.(
2,
6,
10) We conducted this study to determine whether TIVAPs port placement on the arm would be applicable to other cancer patients as well as breast cancer.
Thrombosis can be very serious and affect the prognosis of cancer patients. These events are closely related to an increased risk of venous thromboembolism among cancer patients.(
3) Unfortunately, there is no prophylaxis to prevent catheter-related VTE among cancer patients, and anticoagulant administration is recommended for high-risk patients with VTE.(
8,
13) In our study, there was no statistically significant difference in the incidence of thrombosis between the arm and chest port groups.
Catheter-related infections are generally reported in 5.3%-13.0% of cases and are also an important variable in determining the prognosis of cancer patients.(
5) Previous studies reported that the arm port had a higher incidence of infection than the chest port, whereas our study showed the opposite result.(
2) As the results show, there was no difference in the incidence of thrombosis or catheter-related complications between the two groups; however, incision-related complications occurred more frequently in the chest port group. Even though the chest port group had a smaller number of patients, the fact that there were more complications related to wounds in the chest port group seems to have many implications. Considering the reason, the port to be inserted into the arm is 6F, and the port to be inserted into the chest is 8F, which is larger in terms of diameter and thickness. According to the access venous size, the incision windows tend to be larger with increasing caliber and thickness of the access port for insertion. In addition, the distance between the port and the skin is closer to in chest group because the subcutaneous fat layer in the chest is thinner than the same layer in the arm, and the pressure on the skin due to protruding ports can also be higher in the chest port insertion group. It is possible that incision-related complications more frequently occurred in the chest port group for these reasons. However, previous studies have shown no difference in the incidence of infection complications between the two groups.(
6,
7) It should be considered that bias in this study may influence its results like differences of cancer type, sex, and age. In any consideration, since successful establishment for port implantation is an important aspect of management in the administration of chemotherapy and can affect the schedule of antineoplastics in the event of complications, particular attention should be paid to wound sutures.
Finally, there are few studies on the use of intravenous access ports for patients with metastatic cancer. Lebeaux
et al.(
14) reported that the incidence of catheter-related infections was high in patients with metastasis or palliative treatment. In addition, there are few studies comparing complications between arm and chest venous access ports among metastatic cancer patients; in our study, location for access port placement was not associated with any difference in incidence of complications among metastatic cancer patients.
This study has two important limitations. First, this is a retrospective study, and so patient selection bias may present. Second, heterogeneity between the two groups of patients could have affected the results of the study.
Go to :





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download