INTRODUCTION
The treatment of the incompetent saphenous vein has been rapidly changed from the conventional stripping to the minimally invasive endovenous treatments. For about 2 decades, the endovenous thermal ablations including radiofrequency ablation (RFA) and endovenous laser ablation (EVLA) have been the key modalities to treat it.(
1,
2) RFA demonstrated statistically significant differences with regard to decreased pain and better global and physical scores in quality-of-life (QOL) measurements.(
3) EVLA was comparable with conventional stripping in terms of the abolition of reflux and improvement in disease‐specific quality of life as well as the earlier return to normal activity.(
4)
In 2015, cyanoacrylate closure (CAC) using the VenaSeal Closure System (Medtronic, Dublin, Ireland), was approved by the US Food and Drug Administration for commercial use in the United States as a nonthermal, nontumescent therapy for the treatment of saphenous insufficiency. Previous prospective clinical trials provided the results on the safety and effectiveness of CAC. The first in human trial reported a 94.7% closure rate at 12 months that remained unchanged at 24 and 36 months.(
5) Recently, five-year extension study of patients from a randomized clinical trial comparing CAC versus RFA for the treatment of incompetent great saphenous veins was reported.(
6) They reported that CAC and RFA were effective in achieving complete target vein closure of the GSV at long-term follow-up, with CAC demonstrating continued noninferiority to RFA. CAC was also associated with sustained improvements in symptoms and quality of life, lower CEAP class, and high level of patient satisfaction without serious adverse events between 36 and 60 months.
However, one of unique complication after CAC was the glue-induced inflammatory reaction which showed pain, heating sensation, itching, induration, erythema, and/or generalized hives. Although the proper mechanism of this complication has not been reported, clinical and pathologic finding demonstrated the complex hypersensitivity and irritation reaction (CHAIR) due to the injected glue.(
7) The purpose of this study is to evaluate the incidence and possible risk factors for the development of CHAIR after CAC procedure.
Go to :

METHODS
We reviewed the clinical outcomes of 100 consecutive patients who underwent CAC procedure. Preoperative risk factors including baseline demographic information, procedure time, treated truncal veins such as great saphenous vein (GSV) and small saphenous vein (SSV), and the access site for the introduction of glue were analyzed.
Patients with symptomatic saphenous vein reflux evaluated at our institution’s vascular laboratory were included in this study. The indications for CAC were clinical grade C2 to C6 with symptoms present. Colorized duplex scanning was performed before the procedure. The CAC procedure was performed with local anesthesia with or without sedation in the operating room. An 18 G angiocatheter was inserted into the saphenous vein under ultrasound guidance. Access sites were chosen according to the extent of refluxed saphenous vein. Access site for GSV treatment was chosen around the knee joint and the lower calf for the SSV treatment. The procedure was performed under manufacturer’s instructions for use (IFU). The most proximal position of catheter was 5 cm distal to the SFJ or SPJ that was measured by electronic caliper-equipped in ultrasound machine. Concomitant phlebectomy was performed in almost all cases. We measured the pain score immediately after the procedure in patients who were not sedated. In sedated patients, we measured the pain score after full recovery from the sedation. Technical success was defined as no patent lumen along the vein segment that the glue was injected.
Patients were followed at 1 week and 3 months after the CAC procedure. At each follow-up visit, the Revised Venous Clinical Severity Score (rVCSS) and quality of life (QoL) was measured with the Aberdeen Varicose Vein Questionnaire (AVVQ). At all subsequent visits, the patients were examined clinically and with duplex scanning. At 1-week after the procedure, duplex scanning was performed to confirm saphenous vein closure and to evaluate any complications. The CHAIR was defined as the presence of pain, heating sensation, itching, and erythema at the glue injected site with or without generalized hives (
Fig. 1).
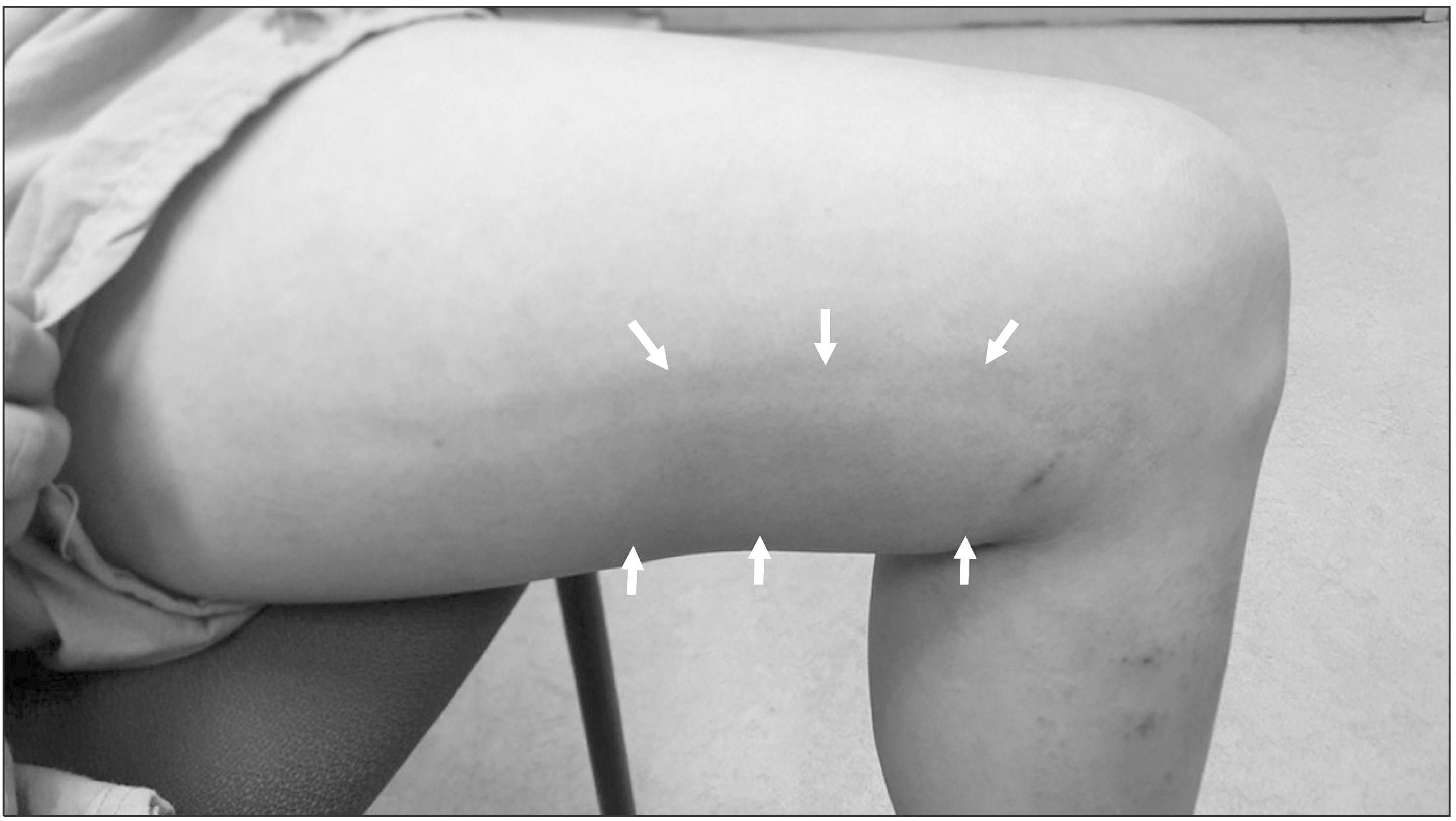 | Fig. 1Typical feature of the complex hypersensitivity and irritation reaction (CHAIR). The patient had a pain, itching and localized heating sensation throughout the treatment site and an erythema and induration was seen along the glue-injected vein. 
|
For the statistical analysis, all data are presented as mean ± standard deviation. Statistical analysis included a two-tailed Fisher’s exact test for categorical values. A two-tailed Student t-test was used to calculate statistical significance for continuous variables. Two groups with CHAIR and without CHAIR were compared with the independent t-test to evaluate the risk factor. SPSS version 22.0 (IBM, Armonk, NY) was used for all statistical analyses. A P value of <0.05 was considered statistically significant.
Go to :

RESULTS
One hundred and ninety saphenous veins were treated in in 100 patients. Sixty-four patients (64%) were female. The mean age was 55.5 ± 12.8 years (19-84).
Table 1 provides procedure details. If the patient had the refluxed saphenous vein in bilateral legs, we treated it in one session simultaneously. One truncal vein treatment in one session was done in 31 patients. Two, three, and four saphenous veins were simultaneously treated in 50, 17, and 2 patients, respectively. Technical success was achieved in all patients. Thrombus extension into the deep vein, so called endovenous glue-induced thrombosis (EGIT), was developed in 9 saphenous veins (9/190, 4.7%) in 9 patients (9%). The incidence of CHAIR was 5 cases (5%).
Table 1
Clinical Outcomes (N = 100)
|
Number (%) |
|
Treated truncal vein with one session |
|
|
1 |
31 (31.0) |
|
2 |
50 (50.0) |
|
3 |
17 (17.0) |
|
4 |
2 (2.0) |
|
Total |
100 (100) |
|
Technical success |
100 (100) |
|
CHAIR |
5 (5.0) |
|
Endovenous glue-induced thrombosis |
9 (9.0) |

All patients with CHAIR were managed by non-steroidal anti-inflammatory drugs (NASID) and/or antihistamines. During the follow-up period, there was no patient with recanalization of the treated saphenous vein. The occlusion rate was 100%. The clinical outcomes obtained immediately after the procedure and at 7 and 90 days postoperatively were improved significantly. The pain score measured by a visual analog scale immediately after the procedure was 1.8 ± 1.4. Most patients experienced pain during the phlebectomy, but not during the CAC procedure. Clinical outcomes are summarized in
Table 2. The pain scores were improved to 0.9 ± 1.4 on postoperative day 7 (P < 0.001). The rVCSS was significantly improved 7 days after the procedure compared with preoperative time (4.7 ± 2.3 vs 1.3 ± 1.3, P < 0.001). The patient QoL score was improved from 13.2 ± 9.2 immediately after the procedure and to 9.1 ± 5.8 on postoperative day 7 (P < 0.001).
Table 2
Clinical Outcomes (N = 100)
|
Mean ± SD |
P value*
|
|
Pain score with visual analog scale |
|
<0.001 |
|
Postoperative 0 |
1.8 ± 1.4 |
|
|
Postoperative 7 |
0.9 ± 1.4 |
|
|
Venous clinical severity score |
|
<0.001 |
|
Preoperative |
4.7 ± 2.3 |
|
|
Postoperative 7 |
1.3 ± 1.3 |
<0.001 |
|
Quality of life score†
|
|
|
|
Preoperative |
13.2 ± 9.2 |
|
|
Postoperative 7 |
9.1 ± 5.8 |
|

We compared the baseline demographics, treated vein, and procedure details of two groups with and without CHAIR (
Table 3). The mean age of patients with CHAIR was significantly younger (56.3 ± 12.3 years vs 39.6 ± 14.0 years, P = 0.004). The other factors including body weight, height, preoperative VCSS and AVVQ were similar in two groups. In addition, the procedure times of two groups were similar. There was no patient with CHAIR after treatment of SSV. The CHAIR was developed after treatment of GSV in all cases. The access site for the introduction of glue was below the knee joint level in all CHAIR cases.
Table 3
Risk Factor of the Complex Hypersensitivity and Irritation Reaction (CHAIR)
|
Risk factor |
Number |
CHAIR (-) |
CHAIR (+) |
P value |
|
Age (years), mean ± SD |
100 |
56.3 ± 12.3 |
39.6 ± 14.0 |
0.004*
|
|
Body weight (kg), mean ± SD |
63 |
65.7 ± 13.9 |
71.5 ± 16.4 |
0.428*
|
|
Height (cm), mean ± SD |
63 |
162.6 ± 9.8 |
168.0 ± 10.8 |
0.292*
|
|
Preoperative VCSS, mean ± SD |
78 |
4.8 ± 2.2 |
3.6 ± 3.9 |
0.422*
|
|
Preoperative AVVQ |
78 |
12.9 ± 9.5 |
12.7 ± 8.3 |
0.974*
|
|
Procedure time (min), mean ± SD |
95 |
76.4 ± 32.8 |
69.8 ± 28.6 |
0.658*
|
|
Treated truncal vein |
190 |
n = 185 |
n = 5 |
|
|
GSV, n (%) |
137 |
132 (71.4) |
5 (100) |
0.008†
|
|
SSV, n (%) |
53 |
53 (28.6) |
0 |
|
|
Access site of GSV |
137 |
n = 132 |
n = 5 |
|
|
Above the knee joint, n (%) |
132 |
132 (100) |
0 |
<0.001†
|
|
Below the knee joint, n (%) |
5 |
0 |
5 (100) |
|

Go to :

DISCUSSION
This study showed that CAC is a safe and effective modality for treatment of incompetent saphenous veins. There were no technical failures. The occlusion of treated saphenous vein was achieved in all patients during the follow-up period. All clinical parameters such as pain score, the VCSS, and the AVVQ were significantly improved. The incidence of EGIT was 9 saphenous veins (9/190, 4.7%) in 9 patients (9%). The incidence of CHAIR, which was the most troublesome complication after CAC procedure, was 5%.
The occlusion rate after CAC procedure was superior compared with RFA or EVLA.(
8) Although limited, the 2-year CAC data are superior. There is negligible difference between RFA and EVLA plots from 6 months onward. Partial and complete recanalization rates were lowest in CAC group throughout the period of follow-up. However, the optimal definition for occlusion has not been reported. If the occlusion was defined as the stump length of <50 mm measured after procedure, the occlusion rate was 90.5% on the postoperative day 7. At the 3- and 6-month postoperative follow-ups, 90.5% and 91.2% of patients had a stump length of <50 mm, respectively.(
9)
Thrombus extension into the deep vein, so called endovenous glue-induced thrombosis (EGIT), may be another worrisome complication of CAC procedure. The incidence of EGIT has been reported from 0% to 21.1%.(
5,
10,
11) The risk factor for the development of EGIT was the smaller diameter of the saphenous vein.(
9) The diameter of GSV in the EGIT (+) group was significantly smaller than that measured for the EGIT (-) group. Also, the peak reflux velocity of the saphenous vein in the EGIT (+) group was significantly lower than that of the EGIT (-) group. Multivariate analysis was performed on the various possible risk factors resulting in EGIT. A saphenous vein diameter of <5 mm was the only significant risk factor.
The clear mechanism of CHAIR has not been released. Tang and Tiwari (
12) reported that abnormal cutaneous erythema was an adverse event thought to be a delayed hypersensitivity reaction to cyanoacrylate. Although it looked like phlebitis, it was generally more widespread, occurring 7-14 days post-procedure with predilection in the great saphenous vein location and in females. The course was self-limiting. Korkmaz
et al.(
13) explained that this reaction occurred with local inflammation after injecting the cyanoacrylate glue. Inflammation is activated when an organism is triggered by stimulants. An acute inflammatory reaction is characterized by neutrophil predominance in the region of the event.(
14) No statistically significant changes were detected between pre- and postoperative counts of either WBC or neutrophils in our study.(
13) Therefore, acute inflammation in the endovenous administration of cyanoacrylate was therefore most likely localized in the vein wall and surrounding tissues. Jones
et al.(
7) reported a severe case with this complication. On postoperative 13 days, the patient complained of leg pain and redness. This was thought to be either phlebitis or an allergic reaction. On postoperative 17 days, she complained of progressive leg pain, chills, and erythema over the medial thigh as a systemic symptom. Histopathologic evaluation of the removed tissue showed intraluminal foreign material and evidence of mononuclear cell inflammation. There was dense chronic inflammation that was localized to the luminal aspect of the vessel. Trichrome elastin and periodic acid-Schiff stains showed the absence of transmural inflammation, specifically with absence of destructive changes toward the periphery of the vessel. Immunohistochemical stain showed that a majority of the mononuclear cells were T lymphocytes, and most of these were of the T4 subset. They concluded that it was the persistent type IV hypersensitivity. However, this complication did not show the recurrence after initial development. Theoretically, hypersensitivity reaction can be developed any time unless the causative organism or foreign body removed from the body.
Typical features of this complication show abnormal skin finding such as erythema, itching, pain, edema, and tenderness over the treated vein area and/or systemic symptom such as fever, chills, generalized hives. Park
et al.(
15) mentioned that this reaction was a phlebitis-like abnormal reaction. Gibson
et al.(
16) analyzed the frequency and severity of this complication. They suggested the hypersensitivity reaction for this complication. Navarro- Triviño
et al.(
2) explained that this complication occurred due to the allergic contact dermatitis. In conclusion, this complication might be developed with contact or irritation of vein after injecting the cyanoacrylate glue. In some patient, hypersensitivity reaction with/without systemic symptoms is developed. Therefore, CHAIR can be the most reasonable terminology.
The risk factors for this complication have been reported variably. Tang and Tiwari (
12) mentioned that the great saphenous vein location and female gender as risk factor according to their experience. Our study showed that the significant risk factors were younger age, GSV treatment, and below-the-knee access site for the introduction of catheter. However, Gibson
et al.(
16) reported that no predictive patient or procedural factors were found to be associated with this complication.
There are several limitations of this study. The results are derived from a small number of patients. All data were collected retrospectively. The laboratory tests including WBC, neutrophil or eosinophil counts as the risk factor analysis were not included in this study.
In conclusion, CAC procedure was an effective and safe modality to treat the saphenous vein insufficiency. The incidence of CHAIR was 5%. The CHAIR occurred when the adhesive was injected at the below-the-knee GSV segment. The mechanical irritation due to knee joint movement might be the possible mechanism for the development of hypersensitivity reaction.
Go to :





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download