NTRODUCTION
Enterovirus is a virus belonging to the Picornaviridae family, consisting of 13 small, non-enveloped, positive single-stranded RNA viruses, of which seven are known as human pathogens. Among them, coxsackievirus, echo virus, numbered enterovirus, and rhinovirus are included in non-polio enteroviruses (NPEVs), excluding three poliovirus serotypes (1). Most NPEVs infections are mild, causing headaches, rhinitis and rash; however, some infections can lead to serious diseases such as cardio myelitis, flaccid paralysis and diarrhoea, particularly in infants (2) and the immunocompromised (3). Enteroviruses are the leading cause of viral aseptic meningitis, and are also implicated in a range of acute infections ranging from non-febrile disease, conjunctivitis and upper respiratory infections, to hand-foot-and-mouth disease (4).
Quercetin-3-glucoside (Q3G) is a natural flavonoid in the form of natural quercetin and exhibits strong antioxidant activity (5). Flavonoids are naturally occurring polyphenol compounds found in many plants and are responsible for various biological functions (6). The pharmacological properties of flavonoids include antioxidants, anti-inflammatory, anticancer, antibacterial and immunomodulatory functions (7). Flavonoids have also been tested for various DNA and RNA viruses. Flavonoids generally act as several mechanisms to block the virus from attaching and entering cells and prevent the virus from assembling or releasing by interfering with translation and multiprotein processing (6).
In this study, we examined the antiviral activity of Q3G against NPEVs. In addition, to further elucidate the action of Q3G on NPEVs proliferation, the effect of Q3G on NPEVs infectivity was investigated through time course experiment, time–of–addition study, real-time PCR analysis.
MATERIAL AND METHODS
Cells, viruses, and chemicals
The EV71 virus was provided from the Division of Vaccine Research of the Korea Center Disease Control and Prevention (KCDC, Cheongwon, Korea), and CVB3 and CVA16 were obtained by Chungcheongnam-do Health and Environment Research Institute in Korea. The viruses were propagated in African green monkey kidney (Vero) cells (ATCC, CCR-81) at 37°C. HRV1B was purchased from ATCC (Manassas, VA, USA), and propagated at 32°C in the human cervical cancer (HeLa) cell line (ATCC, CCR-2). Vero cells and Hela cells were propagated in minimal essential medium (MEM) adding with fetal bovine serum (10% FBS) and antibiotic-antimycotic solution (0.01%). The MEM (Cat NO. 11095080), FBS (Cat NO. 16000044), trypsin-EDTA (Cat NO. 15400054) and antibiotic-antimycotic solutions (Cat NO. 15240096) were purchased from Gibco BRL (Invitrogen Life Technologies, Karlsruhe, Germany). Sulforhodamine B (SRB, Cat NO. S1402) and Q3G were provided by Sigma-Aldrich (St. Louis, MO, USA). Q3G were of HPLC analytical grade with purity of 98%. Tissue culture plates (Cat NO. 353072) were provided from Falcon (BD Biosciences, San Jose, CA, USA). Rupintrivir (Santa Cruz Biotechnology, Dallas, TX, USA, Cat NO. AG7088) was purchased and dissolve
Antiviral activity assay
Antiviral activity was analyzed by the SRB method, as previously reported (8). The day before infection, after seeding of cells in a 96-well plate with 3 × 104 cells per well, the cells cultured for 1 day. The next day, add 10 µL of Q3G to 90 µL of a diluted virus suspension containing the virus stock of Tissue Culture Infectious Dose (TCID50) with 1% FBS and investigate for cytopathic effect (CPE) after 2 days post infection. The antiviral activity of Q3G was determined with four concentrations ranging from 0.4 μg/mL to 50 μg/mL. The cells were propagated at 37°C for 2 days under 5% CO2. After 2 days, the cell supernatant was discarded, washed with PBS, and the culture was terminated. Then, cold acetone was added and incubated at -20°C for 30 minutes to fix the cells. After removing the acetone, the plate was dried for 30 min in a drying oven, after which, 0.4% SRB (w/v) solutions in 1% acetic acid were given to the plates and cultured for 1 h at room temperature. After 1 h, the SRB solution was sucked and the plates were washed with 200 µL of 1% acetic acid. After drying of overnight, the bounded SRB was solubilized by a 10 mM Tris-based solution. The absorbance of the plates was measured at 562 nm using a SpectraMax i3 microplate reader (Molecular Devices, Palo Alto, CA, USA). The results were converted into % of the controls, and the % protection achieved by the experimental compound in the virus-infected cells. The formula was [(ODt)virus − (ODc)virus] ÷ [(ODc)mock − (ODc)virus] × 100%. The (ODt)virus is the optical density (OD) obtained with a given concentration of the experimental compound in virus-infected cells. The (ODc)virus is the OD of the non-experimental compound-treated in virus-infected cells. The (ODc)mock is the OD in normal cells.
Real-Time PCR
The Total RNAs were extracted by an RNA Extraction Mini Kit (Qiagen, Hilden, Germany). Taqman real-time PCR and reverse transcription PCR were conducted using AgPath-ID™ One-Step RT-PCR Reagents (Applied Biosystems, Waltham, MA, USA) on a Bio-Rad CFX96 thermal cycler (Bio-Rad, Hercules, CA, USA). EV71, CVB3 and CVA16 5′ NCR genes and HRV1B 5′ NCR genes were detected using qRT-PCR. The following EV71, CVB3 and CVA16 primers and probe were used: 5′ NCR forward primer 5′- AATCCTGTCACCTCTGACTAAGG-3′, 5′ NCR reverse primer 5′-CATTYTGGACAAAKCGTCTACG-3′, and 5′ NCR probe primer 5′-TGCAGTCCTCGCTCAC-3′. The following HRV1B primers and probe were used: forward primer 5′- AATCCTGTCACCTCTGACTAAGG-3′, reverse primer 5′-CATTYTGGACAAAKCGTCTACG-3′, and probe primer 5′-TGCAGTCCTCGCTCAC-3′. The following GAPDH primers and probe were used: forward primer 5′-GGTCTCCTCTGACTTCAACA-3′, reverse primer 5′-AGCCAAATTCGTTGTCATAC-3′, and probe primer 5′-CCCTCAACGACCACTTTGTCAAG-3′. The cycling conditions were as follows: heating at 45°C for 10 min for reverse transcription, reverse transcription inactivation and initial denaturation condition at 95°C for 10 min, followed by 40 cycles of amplification at 95°C for 15 s and at 60°C for 45 s. The results were obtained using the real-time system AB 7900HT software (Life Technologies, Carlsbad, CA, USA), and all values were normalized to GAPDH levels.
Time course experiment
Vero cells and Hela cells infected with EV71, CVB3, CVA16, and HRV1B at the Tissue Culture Infectious Dose (TCID50) were harvested at the indicated time points including 4, 6, 8 and 10 h post-infection. After adding of Q3G at virus co-infection, total RNA was extracted at the indicated time of post-infection, and the level of 5` non-coding region (5` NCR) gene of viruses was analyzed using real time-PCR.
Time-of-addition assay
TOA analysis was performed to determine the mechanism of action of Q3G. Vero and Hela cells were treated with Q3G of 10 mg/mL at –1 h (1 h before), 0 h (during), or after infection (1, 2, 4, 6, 8 and 10 hours) of virus infection. At 12 h post-infection, virus-specific 5` NCR gene expression was analyzed using real-time PCR.
RESULTS
Antiviral activity and cytotoxicity of Q3G against non-polio enteroviruses (NPEVs)
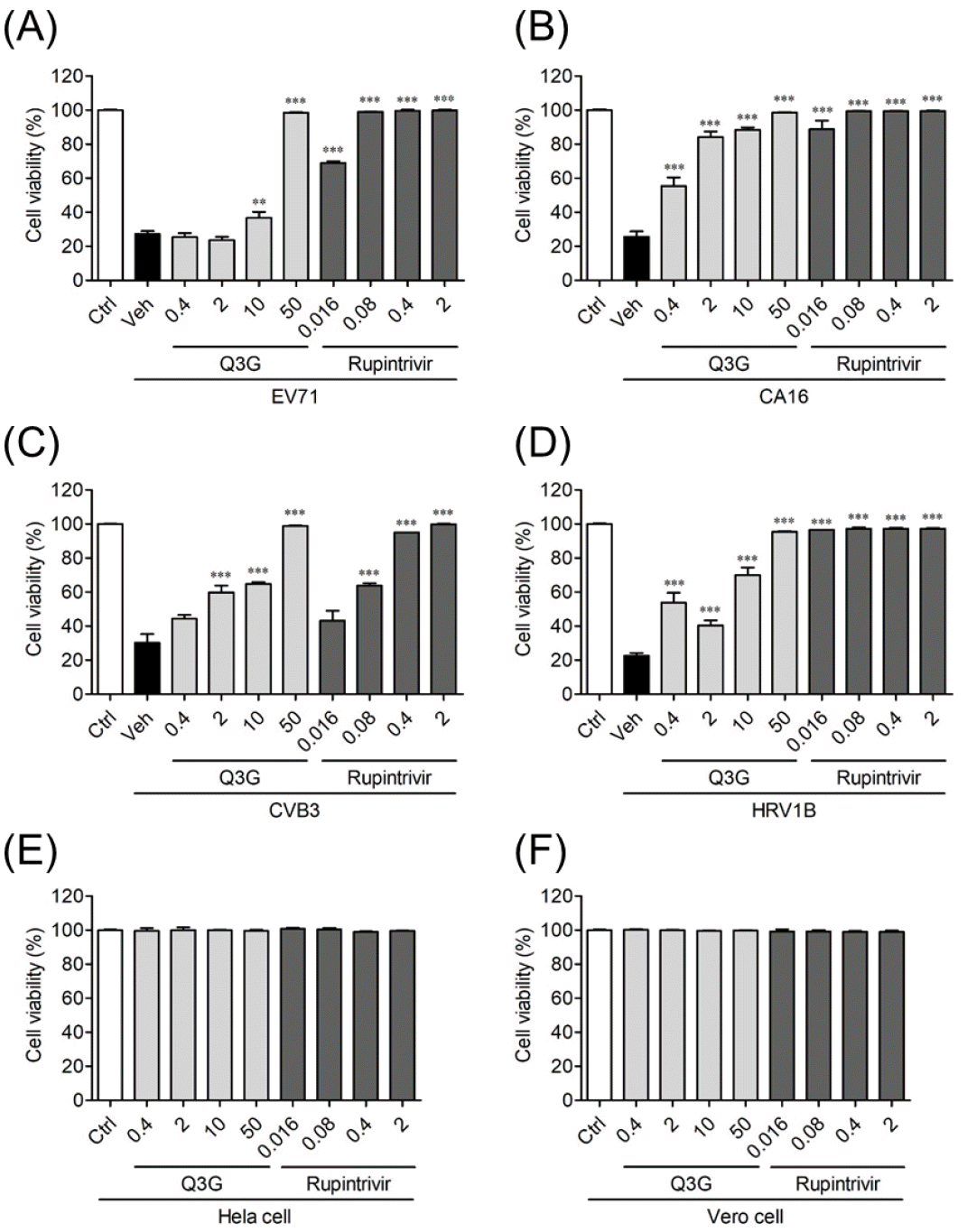
Based on the results of previous studies that Q3G exhibits wide range of antiviral activity against RNA viruses such as zika virus (9) and influenza virus (10), we sought to determine whether Q3G also has antiviral activity against other RNA viruses. Therefore, we tested the antiviral activity against EV71, CVB3, CVA16 and HRV1B belonging to the Picornaviridae family using Q3G. As a result of evaluating virus-induced CPE in cells through SRB analysis, infection with EV71 induced about 80% of apoptosis in Vero cells. However, 50 μg/mL of Q3G inhibited EV71-induced CPE by 100% (Fig. 1A). The IC50 value of Q3G against EV71 was found to be 27.26 μg/mL (Table 1). In Vero cells, CVA16 infection induced about 80% CPE and CVB3 induced about 70% CPE, whereas Q3G at a concentration of 2 μg/mL inhibited CVA16-induced CPE by about 80%, and CVB3 induced CPE about 60% inhibition. Q3G at a concentration of 0.4 μg/mL inhibited CVA16-induced CPE by about 50%, but showed no antiviral activity in CVB3 (Figs. 1B, 1C). In addition, for CVA16 and CVB3, IC50 values were confirmed at the concentrations of 0.74 μg/mL and 6.56 μg/mL, respectively (Table 1). Next, HRV1B induced about 80% of cytotoxicity in Hela cells, but Q3G adding of 0.4 μg/mL inhibited HRV1B-induced CPE by about 50%, thereby exhibiting high antiviral activity (Fig. 1D). The IC50 value of Q3G against HRV1B was found to be 8.01 μg/mL concentration (Table 1). Rupintrivir used as a positive control showed high antiviral activity against all viruses except CVB3 up to the lowest concentration used in the experiment, 16 ng/ml. CVB3 showed about 60% inhibitory activity at a concentration of 80 ng/ml (Fig. 1). The cytotoxicity of Q3G was not observed even at the highest concentration used in the experiment, the highest concentration of 50 μg/mL, for the cytotoxicity of Q3G to Vero cells and Hela cells even at the highest concentration used in the experiment (Figs. 1E, 1F).
Fig. 1
Q3G mediated antiviral activity against NPEVs in vitro. Vero and Hela cells were infected with the TCID50 of (A) EV71, (B) CVA16, (C) CVB3, and (D) HRV1B and treated with indicated concentrations of Q3G and rupintrivir. Cell viability was evaluated via SRB assay, and the results were determined based on absorbance at 562 nm and 650 nm. Bar graphs show mean ± SD. Control (Ctrl) was neither infected nor treated, while vehicle (Veh) was infected with EV71, CVA16, CVB3, and HRV1B but not treated with Q3G and rupintrivir. The cytotoxicity of Q3G was evaluated by SRB assay in (E) Vero and (F) Hela cells. ***P<0.001, based on ANOVA with Bonferroni’s multiple comparison test.
Q3G Inhibited immediate early stage of Viral Infection
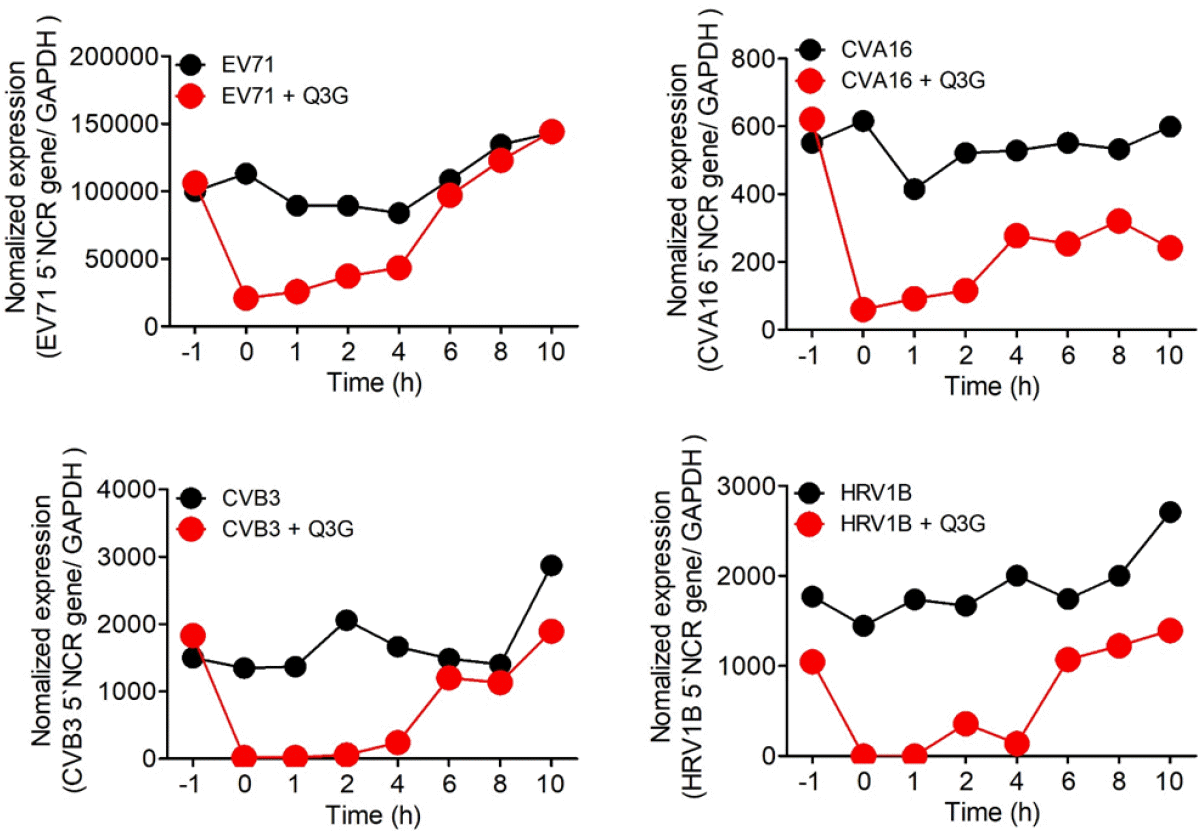
We performed time course and time-of-addition experiments to analyze the Q3G action, antiviral mode of against NPEVs. For the time course experiment, real-time PCR analysis was conducted at 4, 6, 8, and 10 h after the EV71, CVA16, CVB3-infected Vero cells and HRV1B-infected Hela cells which were treated with 10 µg/ml Q3G. The 5' NCR gene of EV71 and CVA16 was detected at 6 hours after infection, and the 5ours after infection. However, Q3G strongly suppressed viral RNA expression up to 10 hours after CVA16, CVB3 and HRV1B' NCR gene of CVB3 was detected at 8 hours after infection. The 5`NCR gene of HRV1B was detected at 4 h infection, while EV71 gene expression until 8 hours after infection (Fig. 2). To investigate which step was affected by Q3G, we performed a time-of-addition experiment in which 10 µg/ml Q3G was added to the culture media at each indicated time of post-infection. And the expression of 5` NCR gene in EV71, CVA16, CVB3 and HRV1B were analyzed at 14 hours after infection. When EV71, CVA16, CVB3 and HRV1B were infected to Vero cells or Hela cells which were treated Q3G before 1 hour, but the virus infection was not inhibited. As a result, it was considered that Q3G did not shows inhibition in the entry stage of EV71, CVA16, CVB3 and HRV1B into the cell. However, when treating Q3G in 1, 2, and 4 hours after infection of EV71, CVA16, CVB3 and HRV1B result shows that viral RNA expression was suppressed in cells (Fig. 3). These results suggest that Q3G suppresses the immediate early stage of the cycle of viral replication.
Fig. 2
The time course of EV71, CVA16, CVB3, and HRV1B infection. Vero and Hela cells infected with the TCID50 of (A) EV71, (B) CVA16, (C) CVB3, and (D) HRV1B were harvested at the indicated time points after 10 mg/ml Q3G had been added. Total RNA was isolated and RNA of EV71, CVA16, CVB3, and HRV1B was analyzed by RT-qPCR. TCID50, 50% cell culture infective dose.
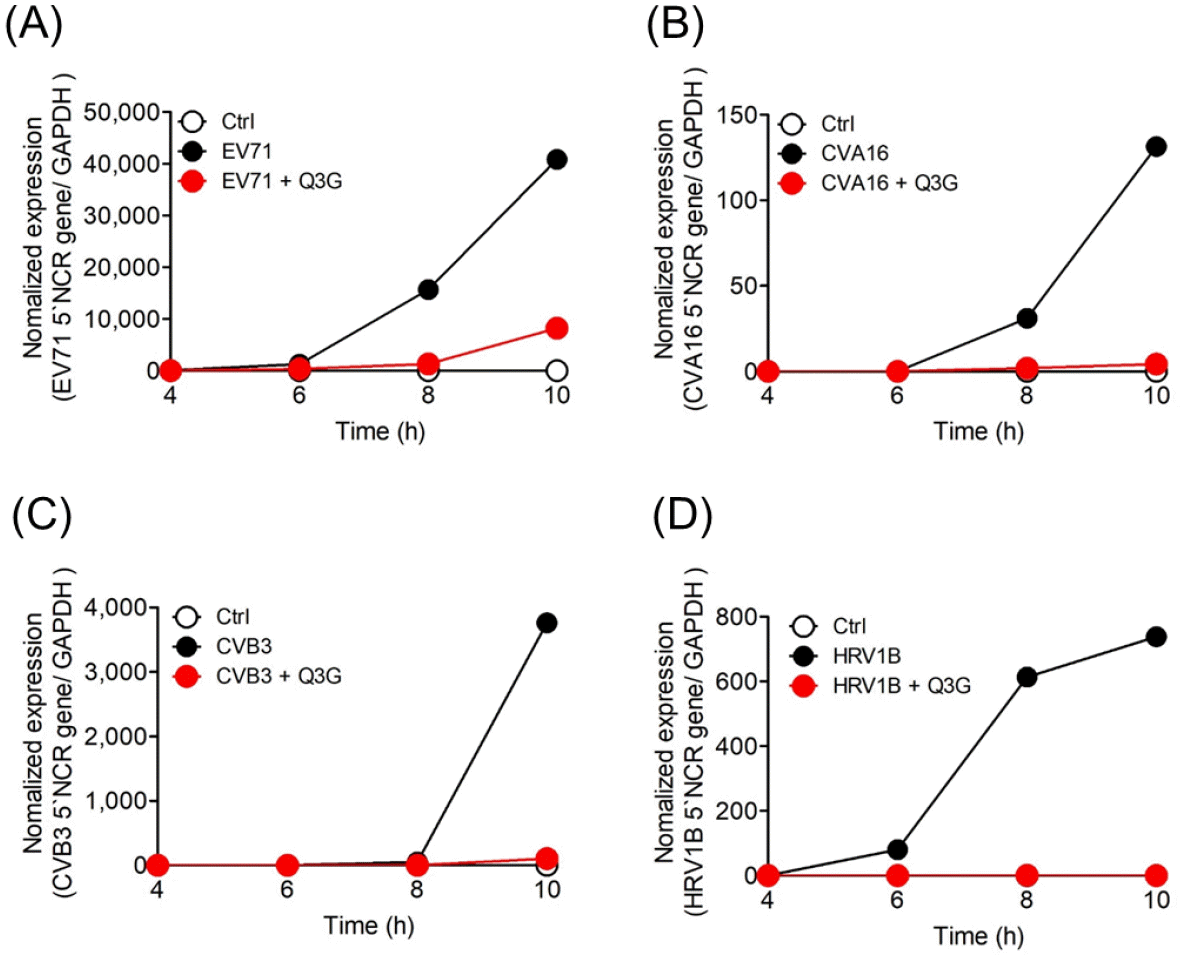
Fig. 3
Time-of-addition experiment testing the effect of Q3G on the EV71, CVA16, CVB3, and HRV1B viral cycle. The cells were infected with the EV71, CVA16, CVB3, and HRV1B, and 10 ug/ml Q3G were also treated with before (-1h), during (0h), or after (1, 2, 4, 6, 8, 10h) at the indicated time points, and the viral mRNA was analyzed 12h post-infection. Total RNA was isolated and viral RNA was analyzed by qPCR.
DISCUSSION
Enterovirus (EV) have emerged as a major human health problem and outbreak epidemics throughout the Asian Pacific region (11). Recently, EV71 has become a rising life-threatening pathogen in several regions (12). EV71 shows extensive tissue tropisms, involving muscle, intestine, and immune cells, and can cause such serious diseases as paralysis, pulmonary edema, or even neurologic symptoms (13, 14).
Antivirals block viral replication and are produced by living organisms or are manufactured chemically (15). Treatment with antivirals can inhibit viral infections and these antivirals are a representative tool to complement the efficacy of vaccines (16). Treating combination of synthetic antivirals with natural antiviral compounds could result in unexpected benefits showing increasing the range of immune response and decreasing the therapeutic dose (17, 18). The synthetic antivirals are designed to inhibit a special step of the virus cycle but reveal a high mutational rate with increasing resistant viral strains (15).
The plant flavonoids were some of the first compositions of plants possessing antiviral efficacy as well as several quinone derivatives (19, 20). Q3G is a polyphenolic compound extracted various plants that possesses antioxidant and anti-inflammatory properties (21). Our study teams reported antiviral activity of various flavonoids against several enteroviruses, influenza virus and porcine epidemic diarrhea virus (22, 23, 24, 25, 26, 27). In this study, Q3G showed high antiviral activity against EV71, CVB3, CVA16 and HRV1B belonging to the Picornaviridae family with non-cytotoxicity at treated maximum concentration (50 μg/mL). Q3G also strongly suppressed virus RNA expression up to 10 hours after CVA16, CVB3 and HRV1B infection, and 8 hours after EV71 infection. Furthermore, pretreatment of Q3G before 1 hour of virus infection didn’t inhibit viral RNA expression and Q3G suppressed viral RNA expression up to 4 hours after infection for EV7, CVB3 and HRV1B, and up to 2 hours after infection with CVA16. These results suggest that Q3G suppresses the immediate early stage of the cycle of viral replication. Therefore, Q3G is a candidate as an antiviral agent for EV71, CVB3, CVA16 and HRV1B.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download