2. Johnson JA, Majumdar SR, Simpson SH, Toth EL. Decreased mortality associated with the use of metformin compared with sulfonylurea monotherapy in type 2 diabetes. Diabetes Care. 2002; 25(12):2244–8.

3. Noto H, Goto A, Tsujimoto T, Noda M. Cancer risk in diabetic patients treated with metformin: a systematic review and meta-analysis. PLoS One. 2012; 7(3):e33411.

4. Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JM. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care. 2009; 32(9):1620–5.
5. Lewis JD, Ferrara A, Peng T, Hedderson M, Bilker WB, Quesenberry CP Jr, et al. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care. 2011; 34(4):916–22.
6. Amshoff Y, Maskarinec G, Shvetsov YB, Raquinio PH, Grandinetti A, Setiawan VW, et al. Type 2 diabetes and colorectal cancer survival: the multiethnic cohort. Int J Cancer. 2018; 143(2):263–8.

7. Zhang ZJ, Zheng ZJ, Shi R, Su Q, Jiang Q, Kip KE. Metformin for liver cancer prevention in patients with type 2 diabetes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2012; 97(7):2347–53.

8. Morales DR, Morris AD. Metformin in cancer treatment and prevention. Annu Rev Med. 2015; 66:17–29.

9. Col NF, Ochs L, Springmann V, Aragaki AK, Chlebowski RT. Metformin and breast cancer risk: a meta-analysis and critical literature review. Breast Cancer Res Treat. 2012; 135(3):639–46.

10. Soranna D, Scotti L, Zambon A, Bosetti C, Grassi G, Catapano A, et al. Cancer risk associated with use of metformin and sulfonylurea in type 2 diabetes: a meta-analysis. Oncologist. 2012; 17(6):813–22.

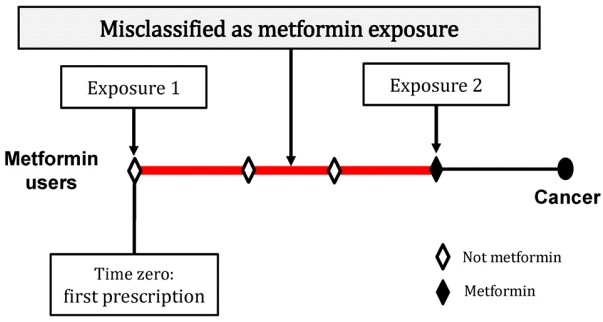
12. Suissa S, Azoulay L. Metformin and the risk of cancer: time-related biases in observational studies. Diabetes Care. 2012; 35(12):2665–73.
14. Hwangbo Y, Kang D, Kang M, Kim S, Lee EK, Kim YA, et al. Incidence of diabetes after cancer development: a Korean national cohort study. JAMA Oncol. 2018; 4(8):1099–105.
15. Mi X, Hammill BG, Curtis LH, Greiner MA, Setoguchi S. Impact of immortal person-time and time scale in comparative effectiveness research for medical devices: a case for implantable cardioverter-defibrillators. J Clin Epidemiol. 2013; 66(8 Suppl):S138–44.

16. Karim ME, Gustafson P, Petkau J, Tremlett H; Long-Term Benefits and Adverse Effects of Beta-Interferon for Multiple Sclerosis (BeAMS) Study Group. Comparison of statistical approaches for dealing with immortal time bias in drug effectiveness studies. Am J Epidemiol. 2016; 184(4):325–35.

17. Agarwal P, Moshier E, Ru M, Ohri N, Ennis R, Rosenzweig K, et al. Immortal time bias in observational studies of time-to-event outcomes: assessing effects of postmastectomy radiation therapy using the national cancer database. Cancer Control. 2018; 25(1):1073274818789355.
19. Sanchez-Rangel E, Inzucchi SE. Metformin: clinical use in type 2 diabetes. Diabetologia. 2017; 60(9):1586–93.

20. Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care. 2006; 29(2):254–8.

21. Bosco JL, Antonsen S, Sørensen HT, Pedersen L, Lash TL. Metformin and incident breast cancer among diabetic women: a population-based case-control study in Denmark. Cancer Epidemiol Biomarkers Prev. 2011; 20(1):101–11.

22. Suissa S. Immortal time bias in observational studies of drug effects. Pharmacoepidemiol Drug Saf. 2007; 16(3):241–9.

23. Lévesque LE, Hanley JA, Kezouh A, Suissa S. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ. 2010; 340:b5087.

24. Jones M, Fowler R. Immortal time bias in observational studies of time-to-event outcomes. J Crit Care. 2016; 36:195–9.

25. Cayea D, Boyd C, Durso SC. Individualising therapy for older adults with diabetes mellitus. Drugs Aging. 2007; 24(10):851–63.

26. Neumiller JJ, Setter SM. Pharmacologic management of the older patient with type 2 diabetes mellitus. Am J Geriatr Pharmacother. 2009; 7(6):324–42.

27. Park SC, Kim DU, Park JE, Kim JJ, Lee SM. Analysis of the correlation between smoking and lung cancer and risk factors for lung cancer in Koreans. Goyang: NHIS Ilsan Hospital National Health Insurance Service Ilsan Hospital;2016.
28. Lai SW, Liao KF, Chen PC, Tsai PY, Hsieh DP, Chen CC. Antidiabetes drugs correlate with decreased risk of lung cancer: a population-based observation in Taiwan. Clin Lung Cancer. 2012; 13(2):143–8.

29. Collaborative Group on Hormonal Factors in Breast Cancer. Type and timing of menopausal hormone therapy and breast cancer risk: individual participant meta-analysis of the worldwide epidemiological evidence. Lancet. 2019; 394(10204):1159–68.

30. Choi IS, Oh CH, Park SY, Ahn SE, Park SJ, Choi HR, et al. Incidence of primary liver cancer in subjects with chronic hepatitis B in Korean national liver cancer screening program. J Liver Cancer. 2017; 17(2):136–43.





 PDF
PDF Citation
Citation Print
Print



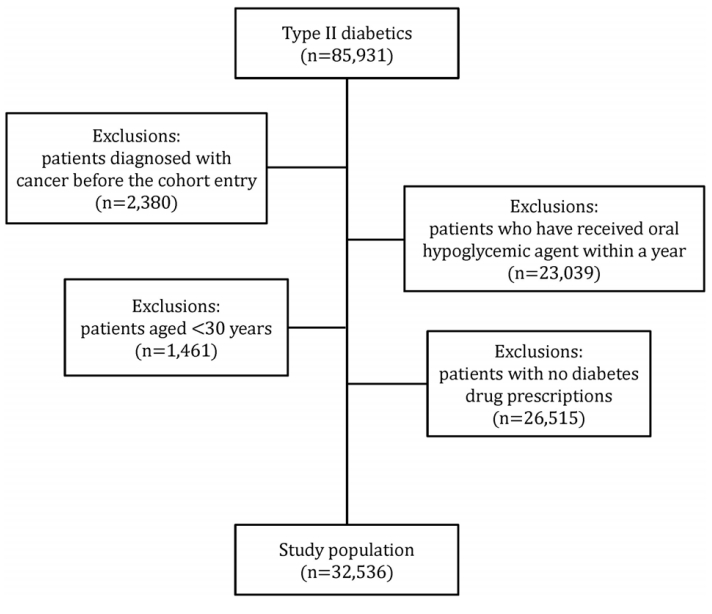
 XML Download
XML Download