INTRODUCTION
CRITERIA FOR PHENOTYPING DPN IN RODENT MODELS ACCORDING TO ‘NEURODIAB’
Behavioural tests
Nerve conduction studies
Peripheral nerve structure
METHODS: LITERATURE SEARCH AND STUDY SELECTION
RESULTS
Summary of included studies and evaluation of studies pre- and post-publication of the Neurodiab guidelines
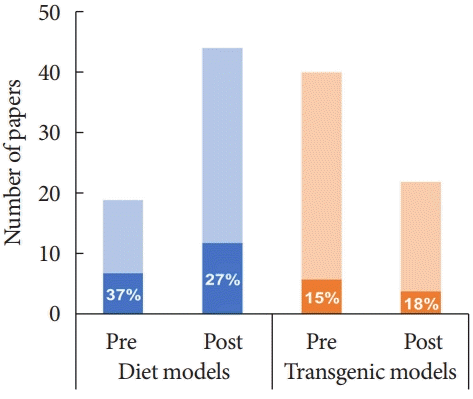 | Fig. 2.Graphical representation of publications examining diabetic peripheral neuropathy (DPN) endpoints in diet+chemically induced (blue) or transgenic (orange) rat models of type 2 diabetes mellitus before (pre) and after (post) the publication of the Neurodiab guidelines for phenotyping DPN in 2014. Dark shading and percentage values indicate the number of studies that assessed 3 DPN endpoints as recommended. |
Table 1.
| Model type | No. of articles included |
Proportion of articles assessing three DPN endpoints |
Articles observing significant change in ≥2 DPN endpoints |
|||
|---|---|---|---|---|---|---|
| Pre-Neurodiab | Post-Neurodiab | Pre-Neurodiab | Post-Neurodiab | |||
| Diet and chemically induced models | ||||||
| SD rat (HFD+low dose STZ) | 51 | 7/18 | 12/33 | 10/18 | 13/33 | |
| Wistar rat (HFD+low dose STZ) | 9 | - | 0/9 | - | 6/9 | |
| Wistar rat (STZ-NA model) | 3 | 0/1 | 0/2 | 1/1 | 2/2 | |
| Total | 63 | 7/19 | 12/44 | 11/19 | 21/44 | |
| Transgenic/spontaneous models | ||||||
| ZDF rat | 36 | 4/20 | 1/16 | 8/20 | 4/16 | |
| BBZDR/Wor rat | 3 | 1/3 | - | 2/3 | - | |
| GK rat | 10 | 0/8 | 1/ 2 | 2/8 | 1/2 | |
| SDT fatty rat | 5 | 0/3 | 0/2 | 2/3 | 0/2 | |
| OLETF rat | 6 | 0/5 | 1/1 | 2/5 | 1/1 | |
| ZDSD-Pco | 1 | 1/1 | - | 1/1 | - | |
| NGR | 1 | - | 1/1 | - | 1/1 | |
| WDF | 1 | 0/1 | - | 0/1 | - | |
| Total | 63-1a | 6/41-1a | 4/22 | 17/41-1a | 7/22 | |
| Grand total | 125 | 13/59 | 16/66 | 28/59 | 28/66 | |
The Neurodiab guidelines were published on the 17th of June, 2014. A 12-month window was included, and thus ‘pre-Neurodiab’ refers to articles published on or before 16th June 2015, and ‘post-Neurodiab’ refers to articles published from 17th June, 2015, to 3rd November 2020.
DPN, diabetic peripheral neuropathy; SD, Sprague-Dawley; HFD, high-fat diet; STZ, streptozotocin; NA, nicotinamide; ZDF, Zucker diabetic fatty; BBZDR, Bio-Breeding Zucker diabetic rat; GK, Goto-Kakizaki; SDT, spontaneously diabetic Torii; OLETF, Otsuka Long-Evans Tokushima Fatty; ZDSD, Zucker diabetic Sprague-Dawley; NGR, Nile grass rat; WDF, Wistar diabetic fatty.
Diet and chemically induced models
Table 2.
| Rat strain/sex |
Diabetes induction paradigm |
Neuropathy phenotype (wk post-STZ) |
|||||
|---|---|---|---|---|---|---|---|
| Diet description | STZ Injection No. & dose, mg/kg | Diabetes duration, wk | Pain related behaviours | Nerve conduction | Peripheral nerve structure | ||
| HFD | |||||||
| SD/male & female [60] | HFD (58% fat) | 1×35 | 2 | m-von Frey (↔0.5, 1, 2) | |||
| Needle-prick (↓0.5, 1, 2) | |||||||
| PWL (radiant heat) (↔0.5, 1, 2) | |||||||
| Cold latency (acetone test) (↔0.5, 1, 2) | |||||||
| Wistar/male [48] | HFD (lard 310 g/kg) | 1×35 | 3 | e-von Frey (↓0, 1, 2, 3) | MNCV (↓3) | ||
| PWL (Hargreaves) (↓0, 1, 2, 3) | |||||||
| Cold water latency (↓0, 1, 2, 3) | |||||||
| SD/male [62] | HFD (22% g fat) | 1×30 | 4 | m-von Frey (↓3, 4) | |||
| PWL (radiant heat) (↓3, 4) | |||||||
| SD/male [63] | HFD (22% g fat) | 1×30 | 5 | m-von Frey (↓3–5) | |||
| PWL (radiant heat) (↓3–5) | |||||||
| SD/male [30] | HFD (24% g fat) | 4×30 (wkly 5–8 wk) | 4 | R/S (↓4) | |||
| PWL (hot plate) (↑4) | |||||||
| SD/male [64] | HFD (24% g fat, lard) | 1×30 | 4 | PWL (Hargreaves) (↑4) | MNCV (↓4) | IENFD (↓4) | |
| SNCV (↓4) | CNFL (↓4) | ||||||
| SD/male [65] | HFD (22% g fat) | 1×30 | 4 | m-von Frey (↓1, 2, 3) | |||
| PWL (radiant heat) (↓1, 2, 3) | |||||||
| SD/male [66] | HFD (22% g fat) | 1×30 | 4 | m-von Frey (↓2, 3) | |||
| PWL (radiant heat) (↓2, 3) | |||||||
| SD/male [50] | HFD (45% kcal fat) | 1×30 | 4 | PWL (Hargreaves) (↑4) | MNCV (↓4) | IENFD (↓4) | |
| SNCV (↓4) | CNFL (↓4) | ||||||
| SD/male [67] | HFD (22% g fat) | 1×30 | 5 | R/S (↓1, 2, 3, 4, 5) | |||
| PWL (radiant heat) (↓1, 2, 3, 4, 5) | |||||||
| SD/male [68] | HFD (60% kcal fat) | 1×45 | 5 | m-von Frey (↓2, 3, 4, 5) | |||
| Wistar/male [69] | HFD (58% fat) | 1×35 | 6 | TFL (radiant heat) (↓6) | |||
| Wistar/male [70] | HFD (30% fat) | 1×30 | 7 | m-von Frey (↓5, 7) | Sciatic-axonal, myelin and Schwann cell injury (7) | ||
| PWL (hot plate) (↓1, 5, 7) | |||||||
| SD/male [49] | HFD (60% kcal fat) | 1×35 | 8 | PWL (hot plate) (↓5, 6, 7, 8) | |||
| Cold water latency (tail-flick) (↓5, 6, 7, 8) | |||||||
| Walking function test performance (↓8) | |||||||
| SD/NR [27] | HFD (lard 310 g/kg) | 2×25 (1 wk apart) | 9 | R/S (↓5, ↑9) | MNCV (↓6) | Sciatic-axonal injury and increased Schwann cells (9) | |
| TFL (hot water immersion) (↓5, ↑9) | |||||||
| SD/male [71] | HFD (58% fat) | 1×35 | 9 | ↓ IENFD (8.5) | |||
| SD/male [72] | HFD (58% fat) | 1×35 | 9 | m-von Frey (↔2, 4, 6.5, 8.5) | ↓ IENFD (8.5) | ||
| Needle-prick (↔2, 4, 6.5, 8.5) | |||||||
| PWL (radiant heat) (↔2, 4, 6.5, 8.5) | |||||||
| Cold latency (acetone test) (↔2, 4, 6.5, 8.5) | |||||||
| SD/female [40] | HFD (60% kcal fat) | 1×30 | 12 | m-von Frey (↓12) | MNCV (↓12) | IENFD (↓12) | |
| PWL (Hargreaves) (↑12) | SNCV (↓12) | CNFL (↓12) | |||||
| SD/male [61] | HFD (45% kcal fat) | 1×30 | 16 | PWL (Hargreaves) (↓15) | MNCV (↓16) | IENFD (↓16) | |
| SNCV (↓16) | CNFL (↓16) | ||||||
| SD/male [52] | HFD (45% kcal fat) | 1×30 | 16 | PWL (Hargreaves) (↑16) | MNCV (↓16) | IENFD (↓16) | |
| SNCV (↓16) | |||||||
| SD/male [42] | HFD (45% kcal fat) | 1×30 | 16 | PWL (Hargreaves) (↑16) | MNCV (↓16) | IENFD (↓16) | |
| SNCV (↓16) | |||||||
| SD/male [43] | HFD (24% g fat, soybean oil and lard) | 1×30 | 16 | ↑ PWL (Hargreaves) (16) | MNCV (↓16) | IENFD (↓16) | |
| SNCV (↓16) | |||||||
| SD/male [41] | HFD (58% kcal fat) | 1×35 | 17 | m-von Frey and R/S (↓17) | ↓ MNCV (17) | Sciatic-axonal injury, lymphocyte infiltration (17) | |
| PWL (hot plate) (↓17) | |||||||
| TFL (hot water immersion) (↓17) | |||||||
| SD/male [56] | HFD (45% kcal fat) | 1×30 | 18 | m-von Frey (↓18) | MNCV (↓18) | IENFD (↓18) | |
| PWL (Hargreaves) (↑18) | SNCV (↓18) | CNFL (↓18) | |||||
| SD/male [57] | HFD (45% kcal fat) | 1×30 | 20 | PWL (Hargreaves) (↑8) | MNCV (↓8) | IENFD (↓20) | |
| SNCV (↓8) | CNFL (↓20) | ||||||
| SD/male [59] | HFD (45% kcal fat) | 1×30 | 20 | PWL (Hargreaves) (↑20) | MNCV (↓20) | IENFD (↓20) | |
| SNCV (↓20) | |||||||
| SD/male [55] | HFD (45% kcal fat) | 1×30 | 28 | PWL (Hargreaves) (↑16, 28) | MNCV (↓16, 28) | IENFD (↓16, 28) | |
| SNCV (↓16, 28) | CNFL (↓16, 28) | ||||||
| HFD measured at multiple time points, in different cohorts | |||||||
| SD/male [51] | HFD (45% kcal fat) | 1×30 | 2-8-16 | PWL (Hargreaves) (↑8, 16) | MNCV (↓2, 8, 16) | IENFD (↓2, 8, 16) | |
| SNCV (↓2, 8, 16) | CNFL (↓2, 8, 16) | ||||||
| SD/male [73] | HFD (45% kcal fat) | 1×30 | 4/16 | PWL (Hargreaves) (↑4, 16) | MNCV (↓4, 16) | IENFD (↓4, 16) | |
| SNCV (↓4, 16) | CNFL (↓4, 16) | ||||||
| SD/male [74] | HFD (45% kcal fat) | 1×30 | 4/16 | PWL (Hargreaves) (↑16) | MNCV (↓16) | IENFD (↓16) | |
| SNCV (↓16) | |||||||
| SD/male [58] | HFD (45% kcal fat) | 1×30 | 8-18-32 | PWL (Hargreaves) (↑18, 32) | MNCV (↓8, 18, 32) | IENFD (↓8, 18, 32) | |
| SNCV (↓8, 18, 32) | CNFL (↓8, 18, 32) | ||||||
| SD/male [54] | HFD (45% kcal fat) | 1×30 | 24/36 | PWL (Hargreaves) (↑8, 24, 36) | MNCV (↓8, 24, 36) | IENFD (↓8, 24, 36) | |
| SNCV (↓8, 24, 36) | CNFL (↓8, 24, 36) | ||||||
| SD/male [75] | HFD (58% fat) | 1×35 | 2 wk/2 mo | m-von Frey (↔2, 4, 6.5, 8.5) | |||
| Needle-prick (↔2, 4, 6.5, 8.5) | |||||||
| PWL (radiant heat) (↔2, 4, 6.5, 8.5) | |||||||
| Cold latency (acetone test) (↔2, 4, 6.5, 8.5) | |||||||
| Wistar/male [76] | HFD | 1×40 | NR | MNCV (↓NR) | |||
| HFHS | |||||||
| SD/male [77] | HFHS (10% oil, 7% sugar) | 1×30 | 3 | m-von Frey (↓3) | |||
| PWL (Hargreaves) (↓3) | |||||||
| SD/male [45] | HFHS (10% lard, 10% sucrose) | 1×35 | 3 | e-von Frey (↓2) | Sciatic-axonal and myelin injury (3) | ||
| SD/male [47] | HFHS (10% lard, 10% sucrose) | 1×35 | 3 | PWL (Hargreaves) (↓2, 3) | |||
| SD/male [44] | HFHS (10% lard, 20% sucrose) | 1×35 | 4 | e-von Frey (↓2, 2.5, 3, 4) | |||
| PWL (radiant heat) (↓2, 2.5, 3, 4) | |||||||
| SD/male [31] | HFHS (10% lard, 20% sucrose) | 1× (30, 35, 40) | 4 | e-von Frey (↓2, 4) | |||
| PWL (radiant heat) (↓2, 4) (only in 35 mg/kg group) | |||||||
| SD/male [46] | HFHS (10% oil, 7% sugar) | 1×30 | 4 | m-von Frey (↓2, 3) | |||
| PWL (Hargreaves) (↓1, 2, 3, 4) | |||||||
| SD/male [78] | HFHS (20% sucrose) | 1×25 | 5 | m-von Frey (↓1, 2, 3, 4, 5) | |||
| Albino/male [79] | HFHS (10% lard, 20% sucrose) | 1×30 | 5 | TFL (radiant heat) (↓5) | Sciatic-myelin injury, increased macrophage and mast cells (5) | ||
| SD/male [37] | HFHS (10% lard, 20% sucrose) | 1×35 | 6 | MNCV (↓6) | |||
| SNCV (↓6) | |||||||
| Wistar/male [38] | HFHS (10% lard, 20% sucrose) | 1×30 | 6 | MNCV (↓6) | Sciatic-axonal and myelin injury (6) | ||
| SNCV (↓6) | |||||||
| SD/male [80] | HFHS (15% lard, 25% sucrose) | 1×25 | 8 | Sciatic-myelin injury (8) | |||
| SD/male [81] | HFHS (10% fat, 70% carb) | 1×40 | 8 | ||||
| SD/male [35] | HFHS (10% lard, 10% sucrose) | 1×30 | 11 | PWL (radiant heat) (↑8) | MNCV (↓11) | ||
| SD/male [36] | HFHS (10% lard, 20% sucrose) | 1×35 | 12 | MNCV (↓12) | |||
| SNCV (↓12) | |||||||
| Wistar/female [82] | HFHS (10% lard, 20% sucrose) | 1×30 | 12 | m-von Frey (↓6) | |||
| SD/male [28] | HFHS (15% lard, 10% white sugar) | 2×25 (1 wk apart) | 16 | MNCV (↓16) | Sciatic-myelinated fiber abnormality (16) | ||
| SD/male [29] | HFHS (15% lard, 10% white sugar) | 2×25 (1 wk apart) | 16 | m-von Frey (↓16) | MNCV (↓16) | Sciatic-myelinated fiber abnormality (16) | |
| PWL (hot plate) (↔16) | |||||||
| SD/male [39] | HFHS (45% kcal as fat) | 1×35 | 2.5 | PWL (hot plate) (↓2.5) | NCV (↔2.5) | ||
| HFHFr | |||||||
| SD/male [83] | HFHFr (660 g fructose; 80 g fat/kg) | 1×35 | 8 | PWL (hot plate) (↓1–8) | Sciatic-axonal injury, reduced Schwann cells (8) | ||
| TFL (hot water immersion) (↓1–8) | |||||||
| SD/male [84] | HFHFr | 1×35 | 4 | e-von Frey (↓2, 3, 4) | |||
| PWL (radiant heat) (↓2, 3, 4) | |||||||
| SD/male [85] | HFHFr (660 g fruc- tose; 80 g fat/kg) | 1×35 | 8 | TFL (hot water immersion) (↓1–8) | Sciatic-axonal injury, reduced Schwann cells (8) | ||
| Formalin-evoked hyperalgesia (tail-flinch) (↑8) | |||||||
| Wistar/male & female [86] | HFHFr (10% fructose solution) | 1×35 | 12 | PWL (hot plate) (↓12) | Sciatic- (12) | ||
| TFL (hot water immersion) (↓12) | |||||||
| Cold water latency (↓12) | |||||||
| Walking function test performance (↓12) | |||||||
| Wistar/male [53] | HFHFr (25.7% g lard, 46.5% g fructose) | 4×25 (2, 8, 14, 20 wk diet) | 36 | m-von Frey (↓–2 to 36) | Sciatic- loss of small and large myelinated fibers (36) | ||
| R/S (↓10–34) | |||||||
| PWL (Hargreaves) (↔0–36) | |||||||
| High caloric diet | |||||||
| SD/male [87] | High-caloric (NR) | 1×30 | 2 | R/S (↓1) | |||
| PWL (radiant heat) (↓1) | |||||||
| SD/male [88] | High-caloric (NR) | 1×30 | 2 | m-von Frey (↓1) | |||
| PWL (radiant heat) (↓1) | |||||||
| STZ/NA models | |||||||
| Wistar/male [33] | Nicotinamide: 1×50 mg/kg IP 15 min | 2 | TFL (radiant heat) (↓2) | Sciatic- axonal and myelin injury, haemorrhage, collagen deposition, vascular degeneration (2) | |||
| Later STZ: 1×52.5 mg/mg IP | Hindpaw cold allodynia (cold water) (↓2) | ||||||
| Tail cold allodynia (cold water) (↓2) | |||||||
| Rotarod (latency to fall) (↓2) | |||||||
| Wistar/male [34] | Nicotinamide: 1×50 mg/kg IP 15 min | 2 | TFL (radiant heat) (↓2) | Sciatic- axonal and myelin injury, haemorrhage, collagen deposition, vascular degeneration (2) | |||
| Later STZ: 1×52.5 mg/kg IP | Hindpaw cold allodynia (cold water) (↓2) | ||||||
| Tail cold allodynia (cold water) (↓2) | |||||||
| Rotarod (latency to fall) (↓2) | |||||||
| Wistar albino [32] | Nicotinamide: 1×110 mg/kg IP 15 min | 5 | PWL (hot plate) (↑1, 2, 3, 4, 5) | Sciatic-nerve cell degeneration (5) | |||
| Later STZ: 1×65 mg/kg IP | Rotarod (latency to fall) (↓1, 2, 3, 4, 5) | ||||||
↑=significant increase, ↓=significant decrease, ↔=no change.
STZ, streptozotocin; HFD, high-fat diet; m-von Frey, manual von Frey; SD, Sprague-Dawley; PWL, paw withdrawal latency; e-von Frey, electronic von Frey; MNCV, motor nerve conduction velocity; R/S, Randal-Selitto; SNCV, sensory nerve conduction velocity; IENFD, intraepidermal nerve fiber density; CNFL, corneal nerve fiber length; TFL, tail flick latency; HFHS, high-fat high-sugar; NR, not reported; NCV, nerve conduction velocity; HFHFr, high-fat high-fructose; NA, nicotinamide; IP, intra-peritoneal.
Diabetes induction approaches
Effects of different T2DM induction methods on DPN phenotypes
1) HFD plus STZ
2) HFHS plus STZ
3) HFHFr plus STZ
4) STZ/NA model
Insulin and lipids in diet and chemically induced models
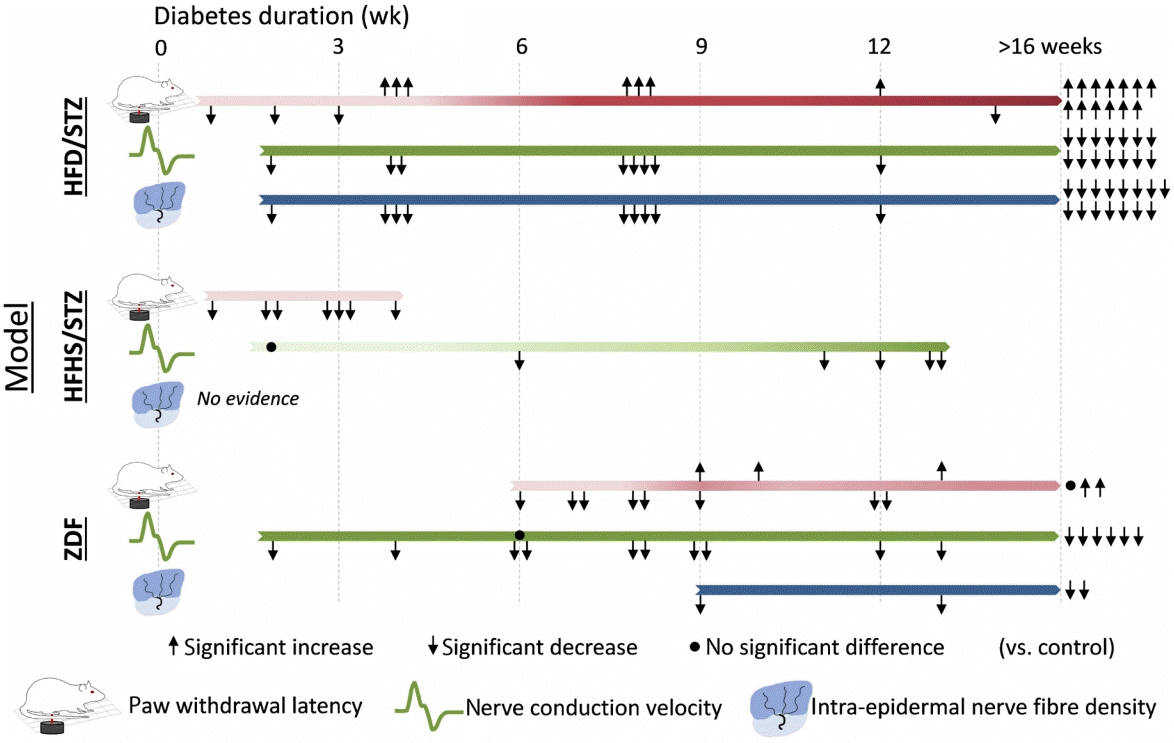 | Fig. 3.Schematic representation comparing evidence for significant changes in key diabetic peripheral neuropathy (DPN) endpoints between diabetes models across disease duration. Arrows indicate the direction of significant change, while dots represent no change. Each data point represents the findings of papers that reported the timepoint at which the assessments were collected using the recommended DPN measures (behaviour assessed by Hargreaves paw withdrawal latency, electrophysiology assessed by motor and sensory nerve conduction velocity and peripheral nerve structure assessed by intraepidermal nerve fibre density). The darkness of the bar indicates the strength of evidence for impairment in the measure vs. control. High-fat high-fructose/streptozotocin (STZ), STZ/nicotinamide, Zucker diabetic Sprague-Dawley, and Nile grass rat were not included as they had ≤1 data point for each of the requisite DPN measures (Tables 2 and 3). Diabetes duration for Zucker diabetic fatty (ZDF) models was calculated as age minus 6 weeks. HFD, high-fat diet; HFHS, high-fat high-sugar. |
Effects of diabetes duration on DPN phenotypes
1) Behavioural tests
2) Nerve conduction studies
3) Peripheral nerve structural analyses
Influence of sex on DPN phenotypes
Methods used for mechanical and thermal sensitivity tests
Transgenic/spontaneous rat models of T2DM
DPN phenotyping in ZDF rats
Table 3.
| Rat strain/sex |
Diabetic animal model details |
Neuropathy phenotype (age in wk) |
||||
|---|---|---|---|---|---|---|
| Age (start-end), wk | Diabetes established, wk | Behavioural assessment of nerve function | Nerve conduction | Peripheral nerve structure | ||
| ZDF | ||||||
| ZDF/male [105] | 5–16 | BGL measured, not diagnostic | m-von Frey (↓6, 12, 14, 16) | |||
| PWL (radiant heat) (↓6, 12, 14, 16) | ||||||
| ZDF/male [106] | 6–17 | BGL measured, not diagnostic | e-von Frey (↓7–8, 12–13, 16–17) | |||
| PWL (Hargreaves) (↑16–17) | ||||||
| ZDF/male [107] | 5–10 | Yes | R/S (↓8–9) | |||
| ZDF/male [100] | 8–39 | BGL measured, not diagnostic | R/S (↓18, 24, 32, 36) | |||
| TFL (↓8, ↑16, 20, 24, 28, 36) | ||||||
| ZDF/male [108] | 8–14 | Yes | e-von Frey (↓10, 14) | MNCV (↓8, 10, 12, 14) | ||
| PWL (radiant heat) (↓8, 14) | SNCV (↓8, 10, 12, 14) | |||||
| ZDF/male [109] | 7–29 | Yes | m-von Frey (↔9, 11, 13, 15, 17, 19, 21, 23, 25, 27, 29) | |||
| ZDF/male [110] | 4–30a | Yes | e-von Frey (↓14a) | MNCV (↓26a) | IENFD (↓30a) | |
| PWL (Hargreaves) (↓18a) Grip-strength (↓16a) | SNCV (↓12.5a) | Sural nerve axonal atrophy (30) | ||||
| Rotarod (latency to fall) (↓14a) | ||||||
| ZDF/male [111] | 5–13 | NR | m-von Frey (↓11, 12, 13) | |||
| PWL (Hargreaves) (↓13) | ||||||
| ZDF/male [101] | 6–40 | BGL measured, not diagnostic | MNCV (↓12, 14, 16, 20, 24, 28, 32, 36, 40) | |||
| ZDF/male [112] | 7–13 | Yes | R/S (↓12, 13) | |||
| e-von Frey (↓12, 13) | ||||||
| ZDF/male [113] | 8–NR | BGL measured, not diagnostic | MNCV (↓12a) | |||
| ZDF/NR [114] | 8–16 | Yes | m-von Frey (↓14, 15) | SNCV (↓12, 14, 15) | ||
| PWL (Hargreaves) (↓12, 14, 15) | ||||||
| ZDF/male [115] | 6–14 | Yes | m-von Frey (↓13, 14) | |||
| PWL (hot plate) (↓13, 14) | ||||||
| ZDF/male [116] | 6–15 | BGL measured, not diagnostic | m-von Frey (↓13, 14, 15) | Spontaneous locomotor activity (↔13, 14, 15) | ||
| PWL (Hargreaves) (↓13, 14) | ||||||
| ZDF/male [117] | 7–16 | Yes | m-von Frey (↓13, 14, 15, 16) | |||
| PWL (hot plate) (↓13, 14, 15, 16) | ||||||
| ZDF/NR [118] | 8–16 | Yes | m-von Frey (↓13, 16) | SNCV (↓13, 16) | ||
| PWL (radiant heat) (↓13, 16) | ||||||
| ZDF/male [119] | NR | BGL measured, not diagnostic | R/S (↓13) | |||
| ZDF/male [120] | 13 | NR | Sciatic-tibial nerve demyelination (↑13) | |||
| ZDF/male [121] | 14–23 | BGL measured, not diagnostic | R/S (↓18, 20, 22) | MNCV (↓23) | IENFD (↓23) | |
| TFL (↑14, 16,18, 20, 22) | SNCV (↓23) | Peroneal nerve myelinated fiber density (↑23) | ||||
| Myelin area (↓23) | ||||||
| ZDF/NR [122] | 6–38 | Yes | R/S (↓15) | |||
| m-von Frey (↓15) | ||||||
| Spontaneous locomotor activity (↓7, 15, 31) | ||||||
| ZDF/male [123] | 4–19 | Yes | R/S (↓15, 16, 17, 18) | |||
| PWL (hot plate) (↓15, 17, 18) | ||||||
| Cold hyperalgesia (↑18) | ||||||
| ZDF/male [97] | 5–20 | BGL measured, not diagnostic | MNCV (↓19) | |||
| ZDF/male [124] | 9–19 | BGL measured, not diagnostic | m-von Frey (↓15, 19) | MNCV (↓15, 19) | IENFD (↓15/19) | |
| R/S (↑15, 19) | SNCV (↓15, 19) | Tibial nerve myelin thickness (↓19) | ||||
| PWL (Hargreaves) (↑15, 19) | G-ratio and axon diameter/myelin thickness (↑19) | |||||
| ZDF/male [125] | 14–20 | BGL measured, not diagnostic | m-von Frey (↔16, 18, 20) | |||
| ZDF/male [126] | 6–25 | BGL measured, not diagnostic | m-von Frey (↓25a) | MNCV (↔12a, ↓25a) | IENFD (↓25a) | |
| PWL (Hargreaves) (↔25a) | SNCV (↔12a, ↓25a) | Sural nerve morphometry (↔25a) | ||||
| ZDF/male [127] | 6–40 | Yes | R/S (↓17, 33) | MNCV (↓38–40) | IENFD (↓35) | |
| m-von Frey (↑31, 38) | SNCV (↓38–40) | |||||
| ZDF/male [128] | 7–17 | BGL measured, not diagnostic | MNCV (↓17) | |||
| ZDF/female [129] | 8–18 | BGL measured, not diagnostic | MNCV (↓18) | Sciatic nerve DNA damage and neuronal injury (↑18) | ||
| SNCV (↓18) | ||||||
| ZDF/male [102] | 9–19 | NR | MNCV (↓18, 19) | Sciatic nerve histopathology (↔19) | ||
| NCV (↓18, 19) | ||||||
| ZDF/male [130] | 10–18 | Yes | R/S (↓18) | |||
| PWL (Hargreaves) (↓18) | ||||||
| ZDF/male [131] | 14–22 | BGL measured, not diagnostic | MNCV (↓21, 22) | |||
| ZDF/male [132] | NR | BGL measured, not diagnostic | m-von Frey (↓18, 32) | MNCV (↓NR) | ||
| PWL (Hargreaves) (↑NR) | SNCV (↓NR) | |||||
| ZDF/male [133] | 7–21/27 | BGL measured, not diagnostic | MNCV (↓21/27) | |||
| ZDF/male [134] | 6–24 | NR | PWL (Hargreaves) (↑24) | MNCV (↓24) | ||
| SNCV (↓24) | ||||||
| ZDF/male [135] | 6–28 | NR | PWL (Hargreaves) (↑28) | MNCV (↓28) | ||
| SNCV (↓28) | ||||||
| ZDF/male [99] | 6–35 | BGL measured, not diagnostic | e-von Frey (↔ up to 35) | NCV (↔up to 35) | ||
| TFL (↓35) | ||||||
| ZDSD | ||||||
| ZDSD/male [103] | 16–34 | BGL measured, not diagnostic | PWL (Hargreaves) (↑34) | MNCV (↓34) | IENFD (↔34) | |
| SNCV (↓34) | CNFL (↓34) | |||||
| NGR | ||||||
| African NGR/male [104] | 52–104 | Yes | m-von Frey ↑ | MNCV ↓ | IENFD ↓ | |
| PWL (Hargreaves) ↑ | ||||||
For each rat strain, studies are listed according to age of earliest standardised neuropathic assessment. Number(s) in parentheses refer to the age of rats in weeks, except for where diabetes duration is used and clearly mentioned. ↑=significant increase, ↓=significant decrease, ↔=no change.
ZDF, Zucker diabetic fatty; BGL, blood glucose level; m-von Frey, manual von Frey; PWL, paw withdrawal latency; e-von Frey, electronic von Frey; R/S, Randal-Selitto; TFL, tail flick latency; MNCV, motor nerve conduction velocity; SNCV, sensory nerve conduction velocity; IENFD, intraepidermal nerve fiber density; NR, not reported; NCV, nerve conduction velocity; ZDSD, Zucker diabetic Sprague-Dawley; CNFL, corneal nerve fiber length; NGR, Nile grass rat.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download