Introduction
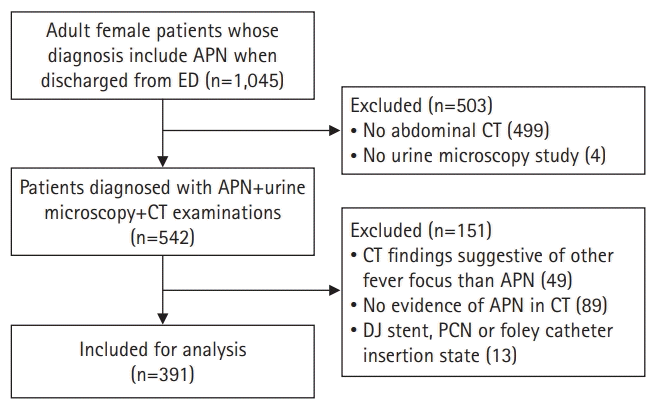
Methods
1. Subjects
2. Outcome measures
3. Statistical analysis
Results
1. General characteristics
Table 1.
2. Comparison of patients with normal (0–5/HPF) and abnormal WBC count (>5/HPF) in urine microscopic study
Table 3.
Values are presented as mean ± standard deviation or number (%).
WBC, white blood cell; HPF, high power field; APN, acute pyelonephritis; CVA, costovertebral angle; UTI, urinary tract infection; NLR, neutrophil-to-lymphocyte ratio; CRP, C-reactive protein; ESBL, extended-spectrum β-lactamase; CT, computed tomography.
3. Logistic regression analysis
Table 4.
| WBC of >5/HPF | OR (95% CI) | p-value | Adjusted OR (95% CI) | p-value |
|---|---|---|---|---|
| Previous antibiotics use | 0.249 (0.140–0.441) | <0.001 | 0.249 (0.138–0.45)a) | <0.001 |
| Fever | 1.814 (0.816–4.035) | 0.144 | 1.916 (0.821–4.469)a) | 0.133 |
| Flank pain or back pain | 0.838 (0.520–1.351) | 0.469 | 1.033 (0.618–1.727)a) | 0.900 |
| Dysuria | 1.063 (0.647–1.745) | 0.809 | 1.089 (0.63–1.883)a) | 0.760 |
| Frequency | 1.240 (0.769–2.000) | 0.378 | 1.176 (0.7–1.976)a) | 0.539 |
| Residual urine sense | 0.785 (0.461–1.339) | 0.375 | 0.904 (0.507–1.611)a) | 0.731 |
| Urgency | 1.573 (0.851–2.907) | 0.149 | 1.513 (0.787–2.908)a) | 0.214 |
| CVA tenderness | 0.969 (0.580–1.620) | 0.905 | 1.105 (0.639–1.912)a) | 0.721 |
| WBC (×1,000/µL) | 1.05 (0.995–1.108) | 0.073 | 1.054 (0.996–1.115)a) | 0.068 |
| Neutrophil (%) | 1.038 (1.009–1.068) | 0.009 | 1.033 (1.003–1.065)a) | 0.030 |
| NLR | 1.059 (1.018–1.102) | 0.005 | 1.051 (1.009–1.094)a) | 0.017 |
| Lactate | 1.140 (0.659–1.973) | 0.639 | 1.04 (0.595–1.817)a) | 0.891 |
| CRP | 1.021 (0.992–1.051) | 0.164 | 1.014 (0.984–1.045)a) | 0.374 |
| Procalcitonin | 1.001 (0.977–1.025) | 0.960 | 1.001 (0.975–1.026)a) | 0.964 |
| Abscess formation or emphysematous change | 0.457 (0.193–1.084) | 0.075 | 0.511 (0.201–1.301)a) | 0.159 |
| Ureteritis | 2.157 (1.301–3.578) | 0.003 | 2.244 (1.328–3.789)b) | 0.003 |
| Unilateral ureteritis | 1.574 (0.928–2.672) | 0.093 | 1.645 (0.95–2.848)b) | 0.076 |
| Bilateral ureteritis | 9.445 (2.226–40.072) | 0.002 | 9.663 (2.239–41.698)b) | 0.002 |
| Cystitis | 2.519 (1.413–4.490) | 0.002 | 2.412 (1.334–4.36)b) | 0.004 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download