Abstract
Background
This study was performed to evaluate periprocedural factors, complications, and repuncture rate of the newly developed puncture needle and compare it with the routinely used puncture needle for ultrasound (US)-guided paracentesis.
Methods
We retrospectively identified 137 patients who underwent US-guided paracentesis between July 2018 and March 2019. Among them, 82 patients underwent US-guided paracentesis with a newly developed puncture needle. The other 55 patients underwent US-guided paracentesis with a routinely used puncture needle. The periprocedural factors, complications, and repuncture rate were compared between the two groups using the Mann-Whitney U test and Fisher exact test. The repuncture-associated factors were assessed using logistic regression analysis.
Results
There were no major or minor complications in either group. The rate of repuncture was significantly lower in the group using the newly developed puncture needle compared with the group using the routinely used puncture needle (p=0.01). The duration of the procedure was significantly shorter with the newly developed puncture needle compared with the routinely used puncture needle (p=0.01). In univariate analysis, the thickness of the abdominal wall (p=0.04) and the use of the newly developed puncture needle (p=0.01) were significantly associated with the rate of repuncture. In multivariate analysis, only the use of the newly developed puncture needle was significantly associated with the rate of repuncture.
Ascites is the presence of abnormal fluid in the abdominopelvic cavity. Approximately 75% of patients with ascites have liver cirrhosis [1]. Ascites is a common complication in patients hospitalized with liver cirrhosis [2,3]. Non-cirrhotic causes of ascites include malignancy, tuberculosis, and pancreatic ascites [4]. In such cases, ascites drainage is important for diagnosing the cause.
Paracentesis is classified as diagnostic or therapeutic based on its purpose. In most cases, diagnostic paracentesis is performed to assess portal hypertension and infected fluid collection [5]. For the differential diagnosis of ascites, tests for the gross appearance of fluid, cell count correction, white blood cell count, and serum ascites albumin gradient are performed [6-10].
Therapeutic paracentesis is performed to relieve abdominal distension and pain in patients with massive ascites. Generally, over 5 L of fluid is drained for therapeutic paracentesis [11]. Albumin is used for plasma expansion to prevent hypovolemia [12]. Despite the risk of hypovolemia, therapeutic paracentesis should be performed in patients with refractory ascites.
Recently, the frequency of ultrasound (US)-guided paracentesis is higher than that of landmark-guided paracentesis. Based on the complication and success rates, US-guided paracentesis has been recommended [13], regardless of the purpose (diagnostic or therapeutic paracentesis).
At our institute, many punctures are performed under US guidance, several of which require repuncture due to the lack of continuous drainage. Recently, a newly developed puncture needle was introduced with a hard plastic sheath and plastic wings, unlike the previously used puncture needle. The plastic wings were thought to be close to the skin, making it easier to fix the sheath after the puncture.
In this retrospective study, we evaluated the periprocedural factors, complications, and repuncture rate of the newly developed puncture needle in US-guided paracentesis. In addition, we compared it to the routinely used puncture needle for US-guided paracentesis.
Ethical statements: This study was approved by the Institutional Review Board (IRB) of Pusan National University (IRB No: PNUH 1907-012-080), and all patients included in this study provided written informed consent to undergo the use of all puncture needles in ultrasound-guided paracentesis.
We searched the institutional database from July 2018 to March 2019 for patients who had undergone US-guided paracentesis with available records in a case form (discussed later). Among the 234 eligible patients, 97 were excluded because of minimum-to-mild ascites (depth of peritoneal fluid less than 3 cm on US). Finally, 137 patients (95 men, 42 women; median age, 62 years; range, 31–84 years) were included. Among them, 82 patients, classified as group A (57 men, 25 women; median age, 62 years) underwent US-guided paracentesis with a newly developed puncture needle, and other 55 patients, classified as group B (38 men, 17 women; median age, 60 years) underwent US-guided paracentesis with a routinely used puncture needle, with a general sheath, and without plastic wings. The puncture needle type was randomly selected.
We retrospectively analyzed reporting templates for US-guided therapeutic paracentesis. At our institution, the clinical and periprocedural data for all patients who underwent US-guided paracentesis were recorded. The reporting template contained the following items: sex, age, platelet count, prothrombin time (PT), PT-international normalized ratio (INR), type of puncture needle (newly developed vs. routinely used), amount of ascites, the thickness of the abdominal wall, duration of the procedure from puncture to fixation, degree of pain, puncture site, and presence of repuncture. For the amount of ascites and thickness of the abdominal wall, the depth of peritoneal fluid and the distance between the skin and ascites were measured in cm on US before puncture. The degree of pain was assessed on the Likert scale (1–10; 1, no pain; 10, intolerable pain) using a questionnaire following paracentesis. The puncture site was selected based on the location of ascites, depending on the operator’s comfort. If the drainage was not smooth after the puncture, repuncture was performed immediately using the same puncture needle. We also reviewed the medical records for complications associated with US-guided paracentesis and classified them as major and minor based on the Society of Interventional Radiology guidelines [14].
Compared to the routinely used puncture needle (BD Angiocath Plus; Becton Dickinson Medical, Franklin Lakes, NJ, USA), the newly developed puncture needle (3S-A.D.Cath-16 gauge; Dukwoo Medical, Hwaseong, Korea) was longer (50 mm vs. 45 mm). The newly developed puncture needle had a hard plastic sheath and plastic wings. Both puncture needles had the same diameter (16 gauge) and the same method of application (Fig. 1).
All patients underwent US-guided therapeutic paracentesis in the supine position, performed by a single radiologist (SBH, 7 years of clinical experience). Punctures were made at the right lower quadrant and left lower quadrant of the abdomen, and the location targeting the Morrison’s pouch. Subsequently, the fluid was drained, and the metallic introducer needle was removed. Drainage of ascites was performed by fixing the remnant plastic sheath. When using the routinely used puncture needle, gauze packing was performed around the remnant plastic sheath. In the other case, plastic wings were attached to the skin by taping.
For periprocedural factors, categorical and continuous variables were expressed as counts (percentage) and median (range), respectively. We compared the periprocedural factors between the two groups using the Mann-Whitney U test and Fisher exact test and assessed the repuncture-associated factors using logistic regression analysis. For all tests, statistical significance was set at p<0.05. All statistical analyses were performed using the IBM SPSS version 22.0 (IBM Corp., Armonk, NY, USA).
Among the 137 patients, group A underwent US-guided paracentesis with the newly developed puncture needle, and group B underwent US-guided paracentesis with the routinely used puncture needle. The clinical causes of ascites were not significantly different between the two groups (Table 1). The rate of repuncture was significantly lower in group A (6.1%; 95% confidence interval [CI], 2.3–13.8) than in group B (21.8%; 95% CI, 12.8–34.5) (p=0.01). The duration of the procedure was significantly shorter in group A (median duration of 1 minute) than in group B (median duration of 2 minutes) (p=0.03) (Table 2). Other characteristics and periprocedural factors were not significantly different between the two groups.
There were no major or minor complications in either group. The thickness of the abdominal wall (p=0.04) and use of the newly developed puncture needle (p=0.01) were significantly associated with the rate of repuncture in the univariate analysis. In the multivariate analysis, only the use of the newly developed puncture needle was significantly associated with the rate of repuncture (p=0.02) (Table 3).
In the five cases of repuncture where US-guided paracentesis was done with the newly developed puncture needle, all repunctures were performed in patients with carcinomatosis peritonei. Among the 12 cases of repuncture with the routinely used puncture needle, most repunctures were performed in patients with liver cirrhosis (11 of 12). In the cases of US-guided paracentesis with the routinely used puncture needle, repuncture was performed because of kinking of the sheath (11 of 12) or lack of definite penetration of the parietal peritoneum (1 of 12).
US-guided paracentesis is safe and widely used to treat ascites. Recently, a new puncture needle with a hard plastic sheath and plastic wings was developed. Our study showed that the rate of repuncture was significantly lower in group A (6.1%; 95% CI, 2.3–13.8) than in group B (21.8%; 95% CI, 12.8–34.5) (p=0.01). Among the repuncture-associated factors, only the use of the newly developed puncture needle was significantly associated with the rate of repuncture (p=0.02).
The peritoneal cavity is the space between the parietal peritoneum and the visceral peritoneum. The parietal peritoneum lines the abdominal wall and is penetrated in US-guided paracentesis [15]. The parietal peritoneum has elasticity [16], which can prevent penetration of the plastic sheath and result in repuncture. However, the newly developed puncture needle for paracentesis has a hard plastic sheath, which may help penetrate the parietal peritoneum and reduce the kinking of the sheath during drainage. Plastic wings contribute to fixation stability following paracentesis.
Regarding the newly developed puncture needle, all repunctures were performed in patients with carcinomatosis peritonei. Loculated ascites is associated with malignancy and inflammation [17]. Due to the presence of loculated ascites, a large amount of ascites could not be drained, and a repuncture was necessary. Unlike the newly developed puncture needle, most repunctures were performed because of kinking of the sheath with the routinely used puncture needle.
Comparing the characteristics and periprocedural factors between the two groups, the duration of the procedure was significantly lesser in group A (median duration of 1 minute) compared to group B (median duration of 2 minutes) (p=0.03). The longer procedural time associated with the routinely used puncture needle might be due to the relatively frequent repunctures. As the frequency of repuncture increases, the likelihood of complications may increase and may also affect patient satisfaction during or after the procedure.
In our results, the thickness of the abdominal wall was a significant factor associated with repuncture on univariate analysis. The newly developed puncture needle was slightly longer than the routinely used puncture needle, which was advantageous for puncture and drainage. However, there were no significant differences in the thickness of the abdominal wall between the two groups.
No complications occurred in either group. Although many patients with ascites present with increased PT or PT-INR, less than 1% of cases have major complications, such as hemoperitoneum [18,19]. In a previous study reviewing hemorrhagic complications following paracentesis in 4,729 cases, severe hemorrhage occurred in 0.2% of all procedures in patients with liver disease [20]. In addition, US-guided paracentesis is safer than bedside procedures [21,22]. All paracentesis procedures were performed under US guidance in our study, which resulted in no major complications following paracentesis.
Our study had several limitations. First, because this study was retrospective, and we only analyzed patients with therapeutic paracentesis, selection bias may exist. However, this study was consecutive, and we compared the periprocedural factors between the two groups. Second, our results were confined to therapeutic US-guided paracentesis. We did not investigate the efficacy and complications of diagnostic US-guided paracentesis. Generally, continuous drainage following sheath fixation is not necessary for diagnostic paracentesis, which acquires a small amount of ascites. There were no other differences in the procedural techniques between diagnostic and therapeutic paracentesis. The efficacy and complications of this new puncture needle could be comparable to that of the routinely used puncture needle in diagnostic US-guided paracentesis. Third, there were no specific criteria for choosing the type of puncture needle, and the choice was made randomly. A study on the indications for a new puncture needle for paracentesis may be necessary. Finally, the study included a small number of participants. Therefore, further prospective studies with larger sample sizes are necessary.
In conclusion, the new puncture needle with a hard plastic sheath and plastic wings can decrease the rate of repuncture and duration of US-guided therapeutic paracentesis without complications.
Notes
Funding
This study was supported by a 2-year research grant from Pusan National University (PNU 2021-0001).
Author contributions
Conceptualization: IWS, SBH, SK, SYH, HYW; Formal analysis: IWS, SK, SBH, NKL, MRJ; Data curation: IWS, MRJ; Funding acquisition, Project administration, Resources, Supervision: SK; Methodology: IWS, SBH; Visualization, Software: IWS; Investigation: IWS, SK; Validation: SK, SYH, HYW, MRJ; Writing-original draft: IWS; Writing-review & editing: SK, SYH, HYW.
References
1. European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010; 53:397–417.
2. Hwangbo Y, Jung JH, Shim J, Kim BH, Jung SH, Lee CK, et al. Etiologic and laboratory analyses of ascites in patients who underwent diagnostic paracentesis. Korean J Hepatol. 2007; 13:185–95.
3. Ginés P, Quintero E, Arroyo V, Terés J, Bruguera M, Rimola A, et al. Compensated cirrhosis: natural history and prognostic factors. Hepatology. 1987; 7:122–8.
4. Moore KP, Wong F, Gines P, Bernardi M, Ochs A, Salerno F, et al. The management of ascites in cirrhosis: report on the consensus conference of the International Ascites Club. Hepatology. 2003; 38:258–66.
5. Runyon BA, Montano AA, Akriviadis EA, Antillon MR, Irving MA, McHutchison JG. The serum-ascites albumin gradient is superior to the exudate-transudate concept in the differential diagnosis of ascites. Ann Intern Med. 1992; 117:215–20.
6. al Karawi MA, Mohamed AE, Yasawy MI, Graham DY, Shariq S, Ahmed AM, et al. Protean manifestation of gastrointestinal tuberculosis: report on 130 patients. J Clin Gastroenterol. 1995; 20:225–32.
7. Runyon BA; AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update. Hepatology. 2009; 49:2087–107.
8. Poonawala A, Nair SP, Thuluvath PJ. Prevalence of obesity and diabetes in patients with cryptogenic cirrhosis: a case-control study. Hepatology. 2000; 32(4 Pt 1):689–92.
9. DeSitter L, Rector WG Jr. The significance of bloody ascites in patients with cirrhosis. Am J Gastroenterol. 1984; 79:136–8.
10. Runyon BA, Akriviadis EA, Keyser AJ. The opacity of portal hypertension-related ascites correlates with the fluid’s triglyceride concentration. Am J Clin Pathol. 1991; 96:142–3.
11. Moore KP, Aithal GP. Guidelines on the management of ascites in cirrhosis. Gut. 2006; 55(Suppl 6):vi1–12.
12. Evans TW. Review article: albumin as a drug. Biological effects of albumin unrelated to oncotic pressure. Aliment Pharmacol Ther. 2002; 16(Suppl 5):6–11.
13. Cho J, Jensen TP, Reierson K, Mathews BK, Bhagra A, Franco-Sadud R, et al. Recommendations on the use of ultrasound guidance for adult abdominal paracentesis: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019; 14:E7–15.
14. Omary RA, Bettmann MA, Cardella JF, Bakal CW, Schwartzberg MS, Sacks D, et al. Quality improvement guidelines for the reporting and archiving of interventional radiology procedures. J Vasc Interv Radiol. 2003; 14(9 Pt 2):S293–5.
15. Tirkes T, Sandrasegaran K, Patel AA, Hollar MA, Tejada JG, Tann M, et al. Peritoneal and retroperitoneal anatomy and its relevance for cross-sectional imaging. Radiographics. 2012; 32:437–51.
16. Churg A, Cagle PT, Roggli VL. Tumors of the serosal membranes. Washington, DC: American Registry of Pathology;2006.
17. Hanbidge AE, Lynch D, Wilson SR. US of the peritoneum. Radiographics. 2003; 23:663–84.
18. Runyon BA. Paracentesis of ascitic fluid. A safe procedure. Arch Intern Med. 1986; 146:2259–61.
19. McVay PA, Toy PT. Lack of increased bleeding after paracentesis and thoracentesis in patients with mild coagulation abnormalities. Transfusion. 1991; 31:164–71.
20. Pache I, Bilodeau M. Severe haemorrhage following abdominal paracentesis for ascites in patients with liver disease. Aliment Pharmacol Ther. 2005; 21:525–9.
21. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013; 143:532–8.
22. Patel PA, Ernst FR, Gunnarsson CL. Evaluation of hospital complications and costs associated with using ultrasound guidance during abdominal paracentesis procedures. J Med Econ. 2012; 15:1–7.
Fig. 1.
Puncture needles for paracentesis. (A) Routinely used puncture needle. (B) Newly developed puncture needle. The newly developed puncture needle has a hard plastic sheath (arrow) and plastic wings (arrowhead).
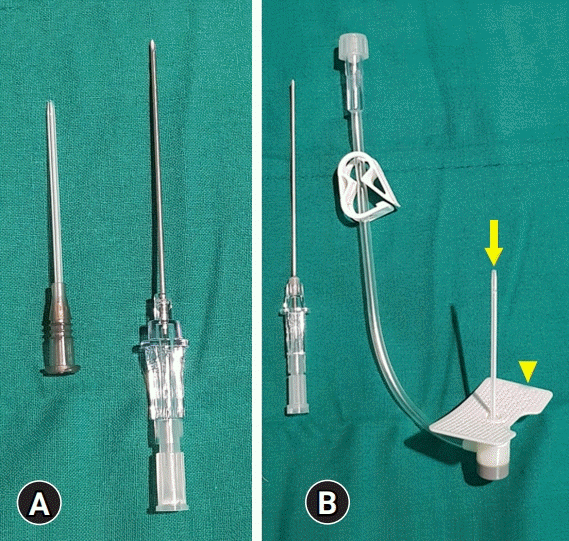
Table 1.
The clinical cause of ascites in two groups
| Clinical cause of ascites | All patients (n=137) | Group Aa) (n=82) | Group Bb) (n=55) | p-value |
|---|---|---|---|---|
| Liver cirrhosis | 105 | 62 (75.6) | 43 (78.2) | 0.84 |
| Malignancy | 29 | 18 (22.0)c) | 11 (20.0)d) | 0.83 |
| Tuberculosis | 3 | 2 (2.4) | 1 (1.8) | >0.99 |
Table 2.
The characteristics and periprocedural factors of the two groups
| Characteristic and periprocedural factor | Group Aa) | Group Bb) | p-value |
|---|---|---|---|
| No. of cases | 82 | 55 | NA |
| Age (yr) | 62 (39–84) | 60 (31–84) | 0.19 |
| No. of women | 25 (30.5) | 17 (30.9) | >0.99 |
| Platelet count before procedure | 127,500 | 146,000 | 0.32 |
| (24,000–380,000) | (45,000–367,100) | ||
| PT-INR | 1.21 (0.9–16.3) | 1.16 (0.91–16.3) | 0.22 |
| Amount of ascites (cm) | 8.0 (3.0–12.0) | 7.0 (3.0–15.3) | 0.82 |
| Thickness of abdomen (cm) | 2.0 (0.9–3.7) | 2.0 (0.7–3.6) | 0.42 |
| Duration of procedure (min) | 1.0 (1.0–4.0) | 2.0 (1.0–6.0) | 0.03 |
| Degree of pain | 3 (1–7) | 3 (1–4) | 0.87 |
| Presence of repuncture | 5 (6.1) | 12 (21.8) | 0.01 |
| Puncture site | 0.47 | ||
| Right lower quadrant | 63 (76.8) | 47 (85.5) | |
| Left lower quadrant | 8 (9.8) | 4 (7.3) | |
| Morrison’s pouch | 11 (13.4) | 4 (7.3) |
Table 3.
Clinical and periprocedural factors associated with repuncture
| Clinical and periprocedural factor |
Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| Odds ratio | p-value | Odds ratio | p-value | |
| Age | 0.97 | 0.24 | ||
| Sex | 1.70 | 0.32 | ||
| Amount of ascites | 0.85 | 0.85 | ||
| Thickness of abdomen | 2.30 | 0.04 | 2.10 | 0.06 |
| Device | ||||
| Routine | 1a) | 1a) | ||
| Hard plastic sheath | 0.23 | 0.01 | 0.23 | 0.02 |
| Puncture site | ||||
| Right lower quadrant | 1a) | |||
| Left lower quadrant | 0.62 | 0.66 | ||
| Morrison’s pouch | 1.06 | 0.95 | ||
| Cause of ascites | ||||
| Liver cirrhosis | 1a) | |||
| Malignancy | 2.23 | 0.15 | ||
| Tuberculosis | 0.00 | 0.99 | ||




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download