Abstract
Enteritis cystica profunda (ECP), a rare and benign condition, is defined as the displacement of the glandular epithelium into the submucosa and more profound layers of the small intestinal wall leading to the formation of mucin-filled cystic spaces. ECP frequently occurs in the ileum or jejunum and is associated with diseases such as Crohn disease and Peutz-Jeghers syndrome. ECP also develops in the absence of known pathology. ECP in the duodenum is very rare and mostly occurs without associated conditions. In this report, we present a rare case of ECP without an associated disease, in the second portion of the duodenum distal to the ampulla of Vater and coexisting with lipoma within the polypoid lesion.
Enteritis cystica profunda (ECP), a rare and nonneoplastic condition, is defined as the displacement of the glandular epithelium into the submucosa or more profound layers of the small intestinal wall and characterized by mucin-filled cystic spaces [1]. ECP occurs mainly in the ileum or jejunum and is related to diseases such as Crohn disease and Peutz-Jeghers syndrome [2-14]. ECP also develops without associated conditions [15-20]. ECP in the duodenum is rare and mostly occurs without an associated pathological condition, unlike ECP in the ileum or jejunum [15-18]. So far, there have been only six reports on ECP in Korea [2,3,15-18]. One report described a case of ECP coexisting with lipoma in the duodenal bulb [18]. In the foreign literature, ECP cases are also rare, and only one case of ECP coexisting with lipoma in the ileum has been reported [4-12,19]. The authors detected an approximately 3.5×1.5×1.0 cm elongated polyp in the second portion of the duodenum distal to the ampulla of Vater (AOV), through esophagogastroduodenoscopy, in a patient without an underlying disease. The polyp was removed using endoscopic snare polypectomy. Histopathological examination confirmed the polyp as an ECP with lipoma. Therefore, the authors report this along with the literature review because this is a rare case in which ECP coexists with lipoma, without an associated condition, in the second portion of the duodenum, which is different from the previously reported case [18] with lipoma in the duodenal bulb.
Ethical statements: This study was approved by the Institutional Review Board (IRB) of the Maryknoll Hospital (IRB No: 2021-308), and the requirement for informed consent from the patients was waived by the IRB.
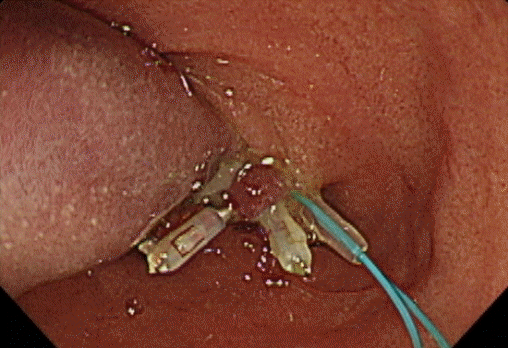
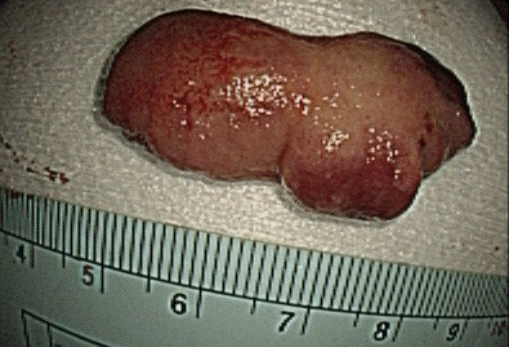
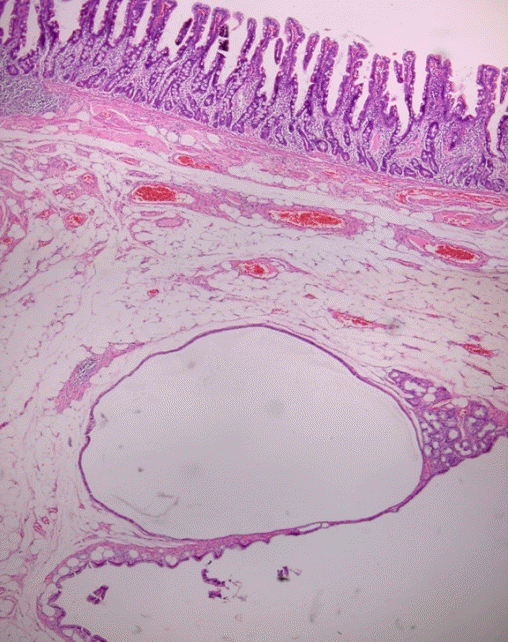
A 78-year-old female without any underlying disease or symptoms underwent esophagogastroduodenoscopy during a health examination. Esophagogastroduodenoscopy revealed an elongated polypoid lesion (3.5×1.5×1.0 cm) with erosions (Fig. 1). The polyp was in the second portion of the duodenum, distal to the AOV. On gross examination, the lower part of the polyp was presumed to be a lipoma, and the upper part to be Brunner gland hyperplasia. Biopsy using cold forceps demonstrated chronic duodenitis with gastric metaplasia. Therefore, she was hospitalized for further examination and polypectomy. There was no history of Crohn disease or Peutz-Jeghers syndrome. Vital signs were as follows: blood pressure, 100/70 mmHg; pulse rate, 72 beats/min; respiration rate, 18 breaths/min; and body temperature, 36.7°C. She had normal consciousness and a healthy appearance. Physical examination revealed normal conjunctivae, anicteric sclerae, and no other abnormal findings were observed. Heart sounds, bowel sounds, and chest auscultation were normal. There was no tenderness or palpable mass on the neck, abdomen, or other lymph nodes. There were no noted findings on limb or neurological examination. In the peripheral blood test, the white blood cell count was 5,800/mm3, hemoglobin was 14.2 g/dL, and platelet count was 279,000/mm3. The results of blood chemistry were as follows: aspartate aminotransferase/alanine aminotransferase, 42/31 IU/L; alkaline phosphatase, 83 IU/L; total protein, 7.6 g/dL; albumin, 4.8 g/dL; total bilirubin, 0.55 mg/dL; amylase, 63 U/L; lipase, 26 U/L; blood urea nitrogen, 20.5 mg/dL; and creatinine, 0.7 mg/dL. The electrolyte values were as follows: sodium, 141 mM/dL; potassium, 4.4 mM/dL; and chloride, 103 mM/dL. The results of the immunoassay test were negative for hepatitis B surface antigen, negative for hepatitis B surface antibody, negative for hepatitis C virus antibody, and serum tumor markers were: alpha-fetoprotein, 3.69 ng/mL; carcinoembryonic antigen, 1.70 ng/mL; and carbohydrate antigen 19-9, 13.98 U/mL. An axial computed tomography image showed focal fat within the duodenum, indicating a lipoma (Fig. 2). Endoscopic snare polypectomy was performed (Figs. 3, 4). Histology confirmed the diagnosis of ECP with a lipoma. Cystically dilated mucosal glands were seen in the submucosa, in which mature adipocytes deposit to produce like mass (Fig. 5). The main component of the polyp was lipoma; therefore, it was presumed that ECP occurred within the lipoma.
Cystica profunda (CP) is a rare condition, defined as the displacement of the glandular epithelium into the submucosa or more profound layers of the gastrointestinal wall, characterized by the formation of benign mucin cysts [1]. When the condition occurs in the colon, it may be termed colitis CP, in the stomach, gastritis CP, and in the small intestine, ECP [1]. The most common type is colitis CP, followed by ECP and gastritis CP [1,4]. ECP develops most frequently in the ileum or jejunum with associated diseases [2-14,19]. However, ECP in the duodenum is the least common and is mostly without causative conditions [15-18]. In Korea, six cases of ECP have been reported [2,3,15-18]. Four cases [15-18] occurred in the duodenum without any associated condition, and the others [2,3] in the ileum or jejunum with Peutz-Jeghers syndrome. One of the four cases demonstrated ECP with lipomas in the duodenal bulb [18]. In the literature of other countries, only one case demonstrating ECP with lipoma in a polypoid lesion that was found on the leading edge of ileocolic intussusception has been reported [19]. It is rare to find ECP in the duodenum without an associated condition and coexisting with lipoma. In the literature of Korea and other countries, this is the third case of lipoma, but in the second portion of the duodenum distal to the AOV, unlike the others in the duodenal bulb or ileum [18,19].
The etiology of CP remains unclear. However, because CP mostly coexists with a specific condition, the condition is presumed to be the etiology [1]. Some common etiologies of CP include inflammatory bowel disease, Peutz-Jeghers syndrome, prolapse, severe infection, ischemia, and trauma [1]. CP is also found in therapeutically irradiated tissues along with surgical anastomosis site and rarely occurs without an etiology [1]. ECP generally develops with Crohn disease and Peutz-Jeghers syndrome, rarely with conditions such as hamartoma, trauma, and primary eosinophilic enteritis, and sometimes without any underlying condition [2-20]. These pathological conditions result in a persistent chronic injury, that, through ulceration and repair, leads to entrapment of glands deep in the intestinal wall [1]. The mechanisms of misplacement may include herniation, implantation after ulceration, mucosal microdiverticula, and reepithelialization of fistulae, and entrapped glands in the intestinal wall can reach the subserosa [1]. Glands entrapped in the intestinal wall commonly undergo dilatation and mucin-filled cystic changes and often have a loss of epithelium due to pressure atrophy [1]. Acellular mucin pools are then left behind and may show calcium deposition or even ossification [1]. This case did not have any associated diseases or lipomas. Based on the etiology, mechanism, and histological results, it is presumed that the lipoma had occurred and underwent persistent chronic injury, leading to the development of ECP.
The cytologic features of the glands in CP are usually bland, and the benign characteristics of CP include the presence of hemosiderin, foreign body giant cells, lack of cytologic atypia, lack of desmoplasia, presence of lamina propria, rounded contours of the epithelial border, and a rim of a single layer of cells along the edge of the mucin pool [1]. In this case, cystically dilated mucosal glands were seen in the submucosa, with deposits of mature adipocytes to produce a mass. The epithelium of the ECP is composed of a mucinous columnar epithelium. In addition, atypical epithelial cells or malignant cells are not seen in ECP. It is not difficult to histologically distinguish ECP from malignancy. To date, it is a predominant view that CP is not a precancerous lesion [1].
CP is detected in the form of a polypoid lesion, through esophagogastroduodenoscopy, gastrointestinal series, endoscopic ultrasound, computed tomography, or magnetic resonance imaging, and can be diagnosed in an excised specimen by surgical operation or endoscopic polypectomy [15]. In this case, an elongated polypoid lesion that was covered with normal surrounding mucosa and erosions on the head was observed endoscopically in the second portion of the duodenum distal to the AOV. When pressed by biopsy forceps, it sank smoothly, showing a positive pillow sign. Therefore, it was presumed to be a lipoma with Brunner gland hyperplasia. After endoscopic snare polypectomy, ECP with lipomas was confirmed by histology.
Patients may be asymptomatic or may present with signs or symptoms of the associated condition or ECP, such as abdominal pain, dyspepsia, diarrhea, bleeding, obstruction, and intussusception [9]. ECP itself does not require specific treatment; however, surgical resection or endoscopic polypectomy can sometimes be performed to resolve the symptoms or to exclude malignant tumors [15]. In this case, the patient had no symptoms or signs. To rule out malignancy, the lesion was removed using endoscopic polypectomy.
The authors report a rare case of ECP in a healthy patient without any particular underlying disease who had a polypoid lesion in the second portion of the duodenum, distal to the AOV, which was suspected to be simple lipoma at first but was histopathologically diagnosed as ECP within lipoma through endoscopic snare polypectomy.
References
1. De Petris G, Leung ST. Pseudoneoplasms of the gastrointestinal tract. Arch Pathol Lab Med. 2010; 134:378–92.
2. Jeong HK, Kim JK, Hwang IS, Kim MY, Kim YM, Lee DY, et al. A case of enteritis cystica profunda in the duodenum. Korean J Gastroenterol. 2002; 39:55–8.
3. Chang HJ, Jung JI, Kim MC, Cho DH, Cho DS, Lee SH, et al. A case of enteritis cystica profunda in the duo-denal bulb. Korean J Gastrointest Endosc. 2005; 31:419–22.
4. You KW, Park SW, Lee GS, Kim du J, Moon HC, Hong GY. A case of enteritis cystica profunda in the am-pulla of vater mimicking choledochocele. Clin Endosc. 2013; 46:178–81.
5. Lee DS, Jeong HR, Kim JO, Tae HJ, Choi HS, Ahn HI, et al. A case of enteritis cystica profunda accompanied by a lipoma in the duodenal bulb. Korean J Med. 2014; 86:314–8.
6. Ahn KS, Bae JD, Kim HG, Shon HS, Choe JY, Kim CH, et al. A case of Peutz-Jeghers syndrome with small bowel perforation and enteritis cystica profunda. Korean J Gastroenterol. 1997; 29:677–82.
7. Aftalion B, Lipper S. Enteritis cystica profunda associated with Crohn’s disease. Arch Pathol Lab Med. 1984; 108:532–3.
8. Kyriakos M, Condon SC. Enteritis cystica profunda. Am J Clin Pathol. 1978; 69:77–85.
9. Dippolito AD, Aburano A, Bezouska CA, Happ RA. Enteritis cystica profunda in Peutz-Jeghers syndrome. Report of a case and review of the literature. Dis Colon Rectum. 1987; 30:192–8.
10. Anderson NJ, Rivera ES, Flores DJ. Peutz-Jeghers syndrome with cervical adenocarcinoma and enteritis cystica profunda. West J Med. 1984; 141:242–4.
11. Alexis J, Lubin J, Wallack M. Enteritis cystica profunda in a patient with Crohn’s disease. Arch Pathol Lab Med. 1989; 113:947–9.
12. Saul SH, Wong LK, Zinsser KR. Enteritis cystica profunda: association with Crohn’s disease. Hum Pathol. 1986; 17:600–3.
13. Mahlow J, Costedio M, Xiao SY, Yuan L, Liu X. Dysplasia involves enteritis cystica profunda in the setting of Crohn’s disease. J Med Cases. 2016; 7:202–7.
14. Zhou T, Chatterjee D, Finch C, Jain S. Peutz-Jegher polyp with enteritis cystica profunda: a neoplastic mimicker. Am J Clin Pathol. 2016; 146(Suppl 1):S6.
15. Ng CF, Hull DA, Feakins RM, Baithun S, Dorudi S. Enteritis cystica profunda. J R Soc Med. 2004; 97:29–30.
16. Dikinis S, Bøhme WP. Enteritis cystica profunda in a patient with Crohn disease. Ugeskr Laeger. 2001; 163:4755–6.
17. Chaturvedi R, Acharya S, Gupte PA, Joshi AS. Coexistent primary eosinophilic enteritis and enteritis cystica profunda. J Postgrad Med. 2012; 58:304–6.
18. Chao JC, Lucha PA Jr. Enteritis cystica profunda: is trauma the etiology? Interval development in the previ-ously normal ileum: a case report and literature review. Mil Med. 2008; 173:513–4.
19. Sugawara Y, Kijima Y, Iizuka H, Endoh G, Okazaki M, Kamita N, et al. A case of adult intussusception due to enteritis cystica profunda of the small intestine. J Japanese Practical Surg Soc. 1994; 55:656–60.
20. Rodríguez-Carrasquel M, Dias CM, Ruiz ME, González O. Enteritis quística profunda: a propósito de un caso. Revista GEN. 2018; 72:25–7.
Fig. 1.
Esophagogastroduodenoscopy findings. The elongated polyp is in the second portion of the duodenum distal to the ampulla of Vater and covered with normal surrounding mucosa. There are erosions (arrows) on the head of the polyp.
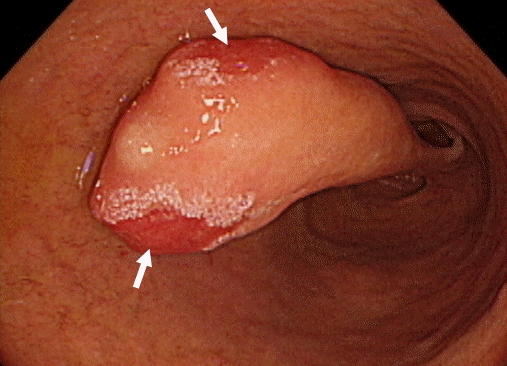
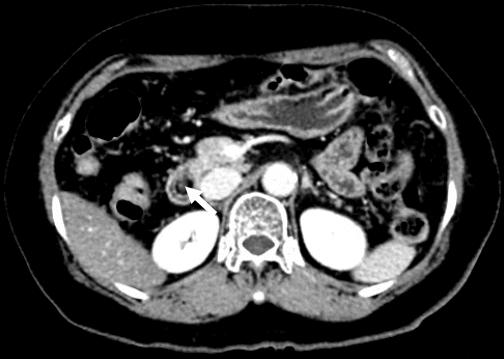
Fig. 2.
Abdominal computed tomography (CT) findings. Axial CT image shows a focal fat-density mass (arrow) in the second portion of the duodenum, which indicates lipoma.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download