INTRODUCTION
Severe abdominal pain occurs mostly in patients with upper abdominal cancers, such as pancreatic cancer, gastric cancer, and liver cancer, at the middle and terminal stages. The therapy methods include palliative tumor treatment, the World Health Organization three-step analgesic ladder, and neurolytic techniques [
1,
2]. Most cancer pain can be relieved by drugs, but approximately 20% of patients cannot achieve satisfactory analgesia with a higher dose of opioid drugs or have a poor quality of life (QOL) due to the serious side effects of the drugs, such as nausea, constipation, and urinary retention [
3].
Blocking the signal transduction of visceral pain has been an effective method for treating upper abdominal pain, and the neurolytic celiac plexus block (NCPB) has been the main technique used since 1914 [
4]. The NCPB has been performed with different clinical techniques according to the puncture route, the site of the needle tip, and the guidance modality (fluoroscopy, computed tomography [CT], ultrasound), including the percutaneous retrocrural, transcrural, or transaortic approaches as well as gastric endoscopic approaches [
5-
7]. A satisfactory therapeutic effect is determined by an ideal drug distribution, but the celiac plexus might be distorted by enlarged tumors or lymph nodes, resulting in an unsatisfactory distribution of the neurolytic agent [
4,
8].
The splanchnic nerve is the main origin of the celiac plexus, which consists of the nociceptive afferent fibers of the upper abdominal parenchymatous organ and is situated posterior to the diaphragmatic crura, piercing the crura of the diaphragm at the T11-12 levels to join the celiac ganglion [
1]. The retrocrural space is a potential closed triangular space, and the effect of splanchnic nerve neurolysis (SNN) is rarely influenced by the surrounding structures [
8]. Several trials have indicated that SNN results in better pain relief, less opioid consumption, fewer complications, and a better QOL compared with the NCPB [
1,
9,
10]. Furthermore, patients have longer pain relief and more satisfaction after SNN compared with NCPB [
11]. The primary puncture method for SNN is the retrocrural or paravertebral approach, guided by fluoroscopy or CT. The paravertebral pathway under fluoroscopic guidance has a risk of pneumothorax and vascular injury [
5]. Thus, SNN under CT guidance has more advantages, and the transdiscal approach could further avoid injury to the paraspinal organs [
12]. However, patients receive a larger dose of radiation under CT guidance, and not all hospitals are qualified to use CT guidance.
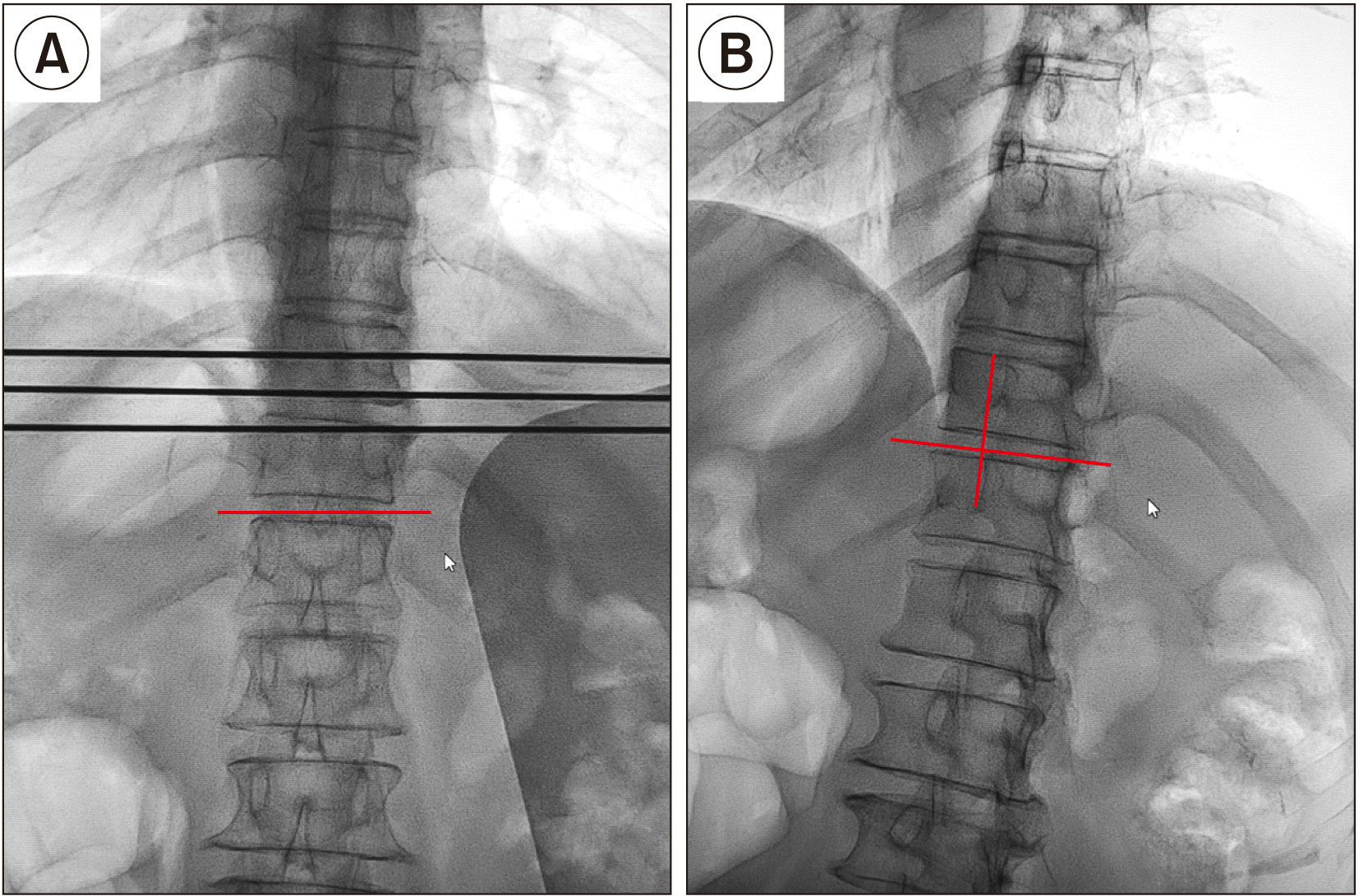
Therefore, the authors aimed to perform SNN via a transdiscal approach under fluoroscopic guidance and observe the feasibility and safety of this technique for treating epigastric cancer pain.
Go to :

RESULTS
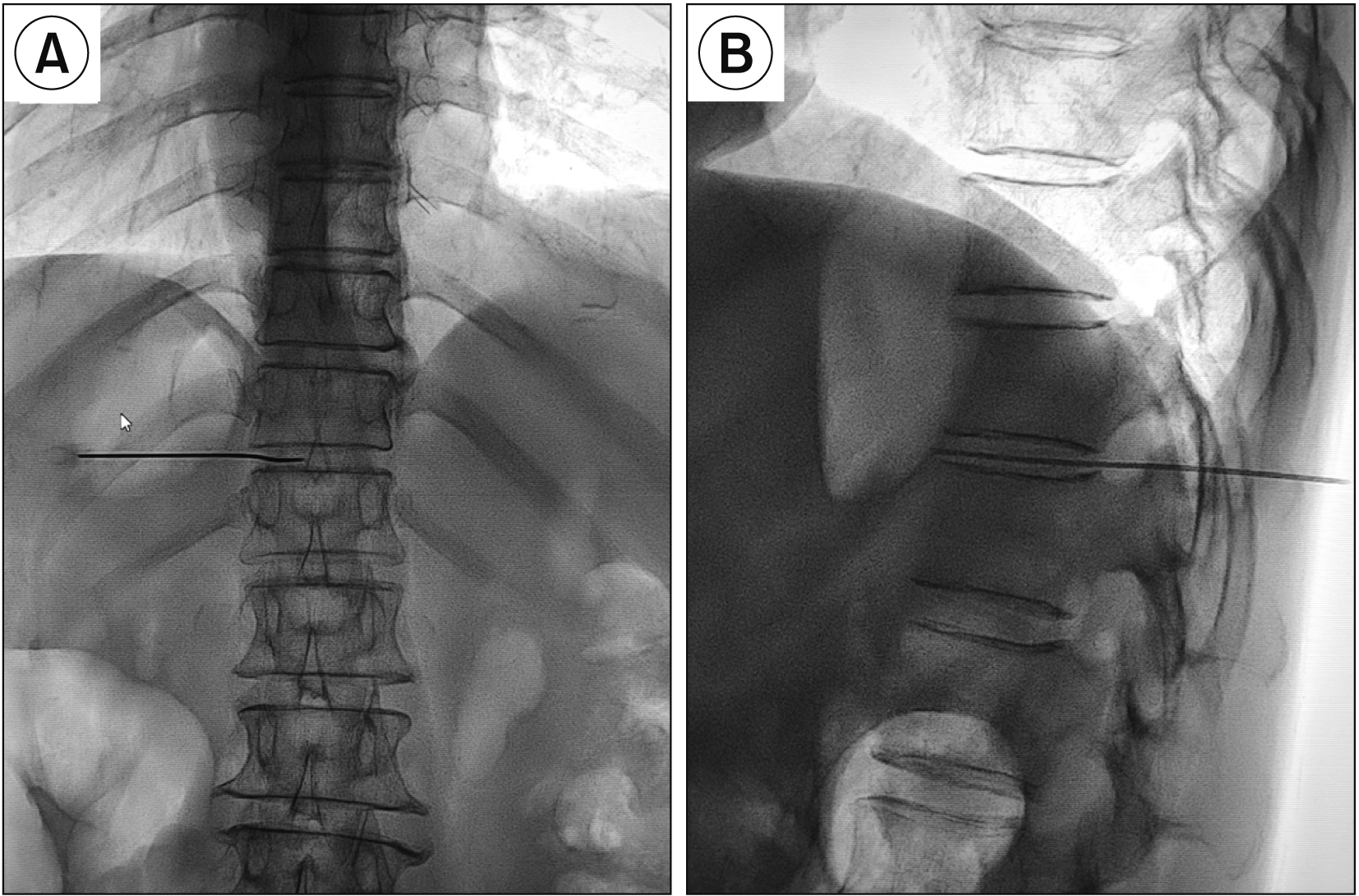
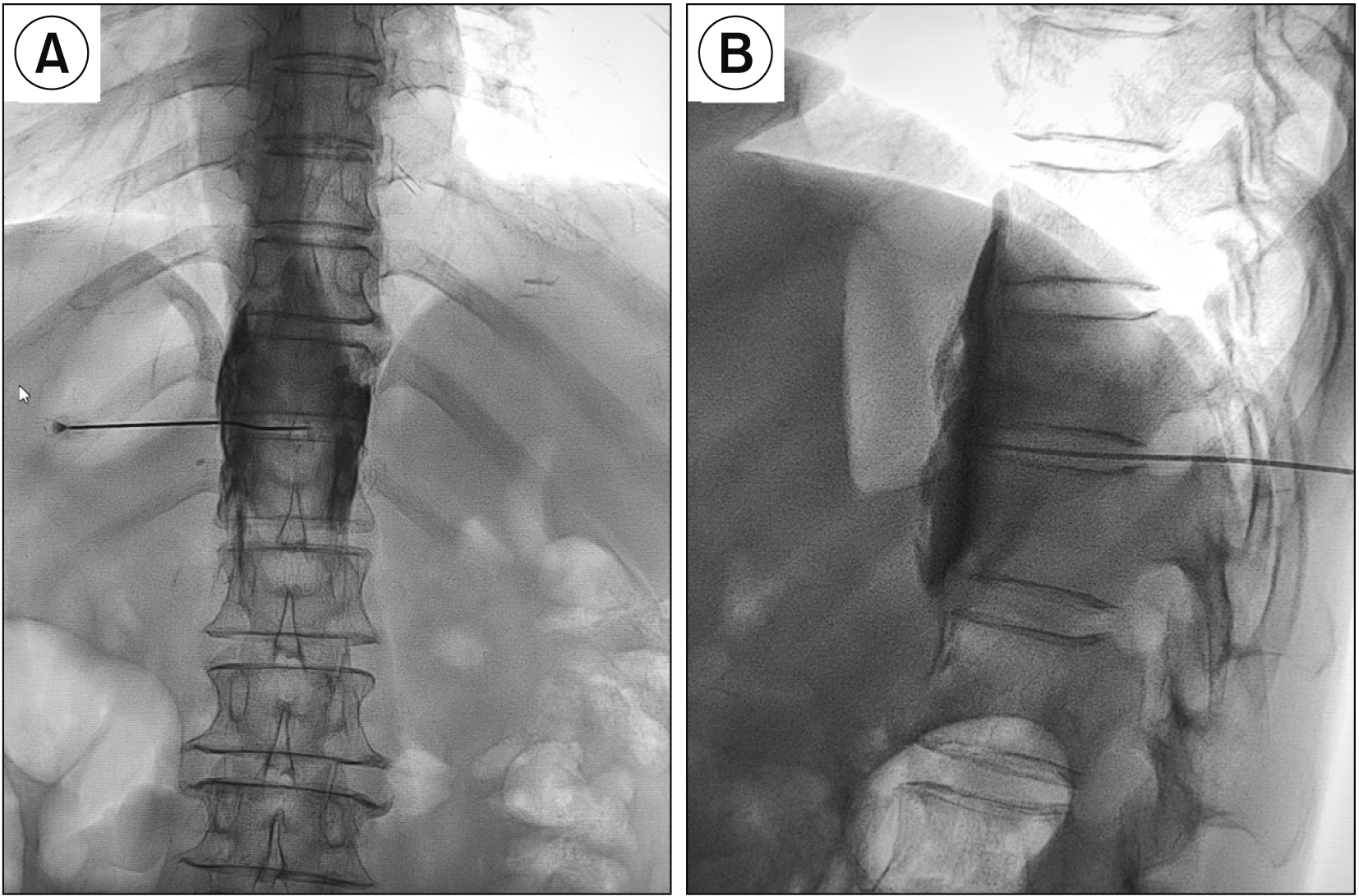
A total of 40 patients were assessed for eligibility. Four patients did not meet the inclusion criteria (mainly due to laboratory test abnormalities or changes in the characteristics of the pain, such as neuropathic and/or somatic pain), and 2 patients declined to take part in SNN due to the potential surgical risks. Thirty-four patients were finally enrolled in the trial. The mean age of the included patients (22 males and 12 females) was 60 ± 12 years (range 48–74 years). The procedures were performed for pain secondary to pancreatic cancer (18 patients), gastrointestinal tract cancer (6 patients), and hepatobiliary cancer (10 patients). Three of the patients had an unsatisfactory effect from the diagnostic splanchnic nerve block, and they only underwent the splanchnic nerve block. Thus, 31 patients underwent both the diagnostic splanchnic nerve block and the SNN, and the number of transdiscal operations was 65. The average operating time was 15.8 ± 5.2 minutes. Seventeen (26.2%) patients had a nonideal distribution of contrast agent, 14 (21.5%) of which were satisfactorily resolved through adjustment of the needle direction, and the other 3 through a second needle puncture, resulting in a single needle success rate of 95.4%.
Compared with the preoperative NRS score, the postoperative NRS score decreased significantly (
Table 1). The NRS score at 1 week after the operation was 2.6 ± 0.7, which was lower than the preoperative score (7.6 ± 2.1) (
P < 0.001), and was maintained at a low level by the final 2 months follow-up. Simultaneously, the postprocedure daily morphine equivalent (mg/d) (1 day, 1 week, 1 month, 2 months) decreased significantly after SNN from 182 ± 36 mg to 52 ± 7 mg (at 1 week) (
Table 1). Two patients even stopped taking morphine 1 week after the SNN until the end of follow-up. Unfortunately, one patient died at 1 month and another at 7 weeks. The QOL and PSS scores increased after SNN, especially after 1 week (
Table 1).
Table 1
Comparison of QOL, PSS,NRS, and daily consumption of morphine from preoperative to postoperative
|
Indexes |
Preoperative
(n = 31) |
Postoperative |
|
1 d (n = 31) |
1 wk (n = 31) |
1 mo (n = 30) |
2 mo (n = 29) |
|
QOL scores |
1.2 ± 1.0 |
5.3 ± 0.8a
|
6.8 ± 1.2a
|
7.2 ± 0.9a
|
7.3 ± 1.1a
|
|
PSS scores |
1.3 ± 0.5 |
6.5 ± 1.1a
|
7.2 ± 1.0a
|
6.8 ± 2.5a
|
7.2 ± 2.1a
|
|
NRS scores |
7.6 ± 2.1 |
3.7 ± 1.0a
|
2.6 ± 0.7a
|
2.8 ± 0.6a
|
2.9 ± 0.8a
|
|
Daily consumption of morphine (mg/d) |
182 ± 36 |
90 ± 14a
|
52 ± 7a
|
60 ± 11a
|
58 ± 9a
|

No serious complications occurred in any patient, including shock, intractable hypotension, pneumothorax, discitis, transient paresthesia, and paraplegia. Three patients (9.7%) suffered from burning pain in the abdomen, which diminished after 24 hours. Four patients (12.9%) suffered from mild diarrhea, which was alleviated in 2–3 days with treatment, including aggressive hydration (oral or parenteral) and antidiarrheal agents. Three patients (9.7%) had postural hypotension, which resolved within 24–48 hours. Five patients (16.1%) suffered from transient backache, which was relieved after taking nonsteroidal anti-inflammatory drugs (
Table 2). No discomfort was found in three patients who underwent the diagnostic block.
Table 2
The complications in patients who underwent splanchnic nerve neurolysis (n = 31)
|
Complication |
Number of cases (%) |
|
Burning pain in the abdomen |
3 (9.7) |
|
Diarrhea |
4 (12.9) |
|
Hypotension |
3 (9.7) |
|
Transient backache |
5 (16.1) |
|
Pneumothorax |
0 |
|
Discitis |
0 |
|
Paresthesia |
0 |

Go to :

DISCUSSION
In this trial, SNN was performed successfully via a novel transdiscal approach under fluoroscopic guidance. The SNN procedure was performed over a shorter operating time, and the patients received less radiation. Meanwhile, the patients felt satisfied, as indicated by the decreased morphine intake and increased PSS and QOL scores. A few patients had transient complications, such as postural hypotension, transient backache and mild diarrhea, from which they recovered quickly. Most importantly, no serious complications occurred, including nerve injury and pneumothorax.
NCPB is an effective therapeutic option for epigastric cancer pain [
14,
15]. Edelstein et al. [
16] reported that among 87 patients who had undergone an NCPB, 35 (40%) patients received a major or complete sustained reduction in pain. However, in advanced malignancies, the celiac plexus anatomy may be distorted by the underlying malignancy or the celiac lymph nodes enlarged; thus, the target of nerve damage can be hard to reach or the spread of neurolytic agents may be limited [
8,
17]. Our data confirmed that the spread of the agent was confined to the front edge of the T11-12 vertebral body and was not affected by the abdominal tumors. Additionally, the performance of an NCPB through a transaortic, retrocrural pathway might lead to complications, including paraplegia, abdominal aortic dissection, renal injury, hematuria, or intravascular injection. Sometimes, NCPB cannot be performed in patients with accompanying severe systemic diseases [
4,
10].
The thoracic splanchnic nerve lies in a small triangular space with well-defined landmarks and boundaries, and has a less variable anatomical relationship with surrounding structures; thus, SNN is easier to perform compared to a conventional NCPB. Süleyman Ozyalçin et al. [
10] found that SNN was an alternative to the NCPB. Ahmed and Arora [
8] also confirmed that SNN was an effective alternative to the NCPB in patients whose celiac anatomy was distorted by tumors. Kapural et al. [
11] performed the NCPB and SNN at different time intervals on the same patient and found that SNN could deliver local anesthetic to the paravertebral compartment medial to the pleural cavity, which was in close proximity to the greater and lesser splanchnic nerves; thus, SNN provided much longer pain relief than the NCPB. Plancarte et al. [
12] proved that SNN could provide analgesia and decrease morphine consumption in patients with upper abdominal malignancies.
SNN is usually performed via a paravertebral approach under fluoroscopic and CT guidance or a transdiscal approach under CT guidance [
12]. The final position of the needle tip in the paravertebral approach lies in the anterior third of the vertebral body on the lateral fluoroscopic view [
1]. The blocking solution is thus more easily spread into the intervertebral foramen, leading to paresthesia and paraplegia and even a high risk of pricking the pleura and paravertebral vessels. Plancarte et al. [
12] proved that the SNN under CT guidance through a transdiscal approach with a unilateral puncture resulted in a bilateral block. However, patients usually receive a large dose of radiation under CT guidance, and not all hospitals are qualified to use CT guidance. Therefore, we performed SNN through the T11-12 intervertebral disc under fluoroscopic guidance. In addition, the curved needle technique made the puncture direction easier to adjust if the drug distribution was unsatisfactory. In the early stage, we used another needle to remedy deficiencies in drug distribution according to previous reports [
8,
11]. After the curved needle was applied with expertise, one needle could complete the operation.
The dose of nerve neurolysis varies greatly in the clinical practice and trials, and the ultimate goal is to achieve complete drug coverage of the involved nerve while avoiding excessive dosage and serious complications. The alcohol dose (approximately 12–20 mL) and concentration (50%–100%) resulting in SNN in clinical studies are often mentioned [
3,
18]. Ahmed and Arora [
8] used 6 mL of 50% alcohol on both sides (a total of 12 mL), while Shwita et al. [
1] and Amr et al. [
19] applied 10 mL of 70% and 100% alcohol, respectively, on each side (a total of 20 mL) to perform the neurolysis. The overdose of alcohol could spread to adjacent structures, and lead to more serious complications, including paresthesia or paraplegia [
20,
21]. In this study, 5 mL contrast agent did not spread to the first half of the vertebral body, and was able to meet the clinical requirement. Usually, higher concentrations of alcohol have a more destructive effect. Higher concentrations and lower doses of alcohol were used in this study to enhance the nerve block effect and reduce complications.
The agents currently used for chemical neurolysis are alcohol, phenol, adriamycin, and methylene blue. Adriamycin mainly acts on the dorsal root ganglia and has a slow onset [
22], while methylene blue has a short acting time. Alcohol acts by denaturing proteins, extracting fatty substances, and precipitating lipoproteins and mucoproteins, damaging both Schwann and nerve cells, and resulting in Wallerian degeneration. Phenol is primarily a local anesthetic at lower concentrations and becomes more neurolytic at higher concentrations [
23], which could produce a block lasting 3–6 months [
3]. Koyyalagunta et al. [
4] found that alcohol and phenol had no difference in complications and pain improvement. In the present study, 99.7% alcohol was chosen, which is safe according to previous literature and can provide a more effective block than other concentrations.
The incidence of mild diarrhea (12.9%), postural hypotension (9.7%), and transient backache (16.1%) in this trial were less than those in a previous study, which was 23%–30%, 19%–50%, and 27%, respectively [
1]. Plancarte et al. [
12] found that SNN via the transdiscal approach had a minor risk of complications (
e.g., pneumothorax) compared with traditional approaches, including the retrocrural and paravertebral approaches, because of the use of a single needle through the disk and a lower volume of neurolytic agent. Simultaneously, the transdiscal approach was a safer route for the needle pathway for avoiding traumatic injury to the arteries branching to the Adamkiewicz artery [
12]. In addition, no opioid withdrawal syndrome was found during this trial, which might be related to a reduced dose of opioids was applied to assist analgesia.
In brief, the main advantages of the transdiscal approach were as follows: (1) it avoids alcohol flow into the intervertebral foramen, which might result in paresthesia and nerve root neurolysis; (2) it avoids injury to the lumbar arteries (which can lead to paraplegia) and other nearby organs, such as the liver, kidney, intestine, pancreas, etc.; and (3) the puncture needle is relatively fixed, and the operation is easy, effective, and safe.
Discitis is the main concern for the transdiscal approach. Recent studies have proven that the risk of discitis is very low, and could be further reduced by the use of prophylactic antibiotics [
20,
23,
24]. Furthermore, no discitis was previously found during SNN and superior hypogastric plexus neurolysis through the intervertebral disc approach [
12,
25]. Although the probability of discitis is low, some measures should be taken, such as decreased puncture times, improved operation skills, and prophylactic antibiotics, to further reduce the risk of this complication.
There are some limitations in our study: (1) Although the punctures were 100% successful, we still required the use of a second needle (3 out of 65) at the early stage of the procedure when the drug distribution was not ideal. (2) This trial had no prospective design, and the sample size was small; thus, the success rate of the puncture and the complication ratio require further observations. Simultaneously, the different effects among different types of cancer were not observed. (3) There was no comparative study between the SNN and traditional NCPB, and the effects of different drugs and different doses of alcohol were not observed in this trial. (4) Although there were fewer complications in this puncture approach, the ultrasound-assisted puncture might be safer, including avoiding the possibility of pleural puncture and vascular injury.
In conclusion, SNN performed through the transdiscal approach under fluoroscopic guidance is a safe and easy operation that has many advantages, including accurate positioning, being a more targeted operation, and producing minimal complications. This method could be popularized to treat abdominal cancer pain.
Go to :





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download