Abstract
Background
Neurokinin-1 (NK1) and calcitonin gene-related peptide (CGRP) play a vital role in pain pathogenesis, and these proteins’ antagonists have attracted attention as promising pharmaceutical candidates. The authors investigated the anti-nociceptive effect of co-administration of the CGRP antagonist and an NK1 antagonist on pain models compared to conventional single regimens.
Methods
C57Bl/6J mice underwent sciatic nerve ligation for the neuropathic pain model and were injected with 4% formalin into the hind paw for the inflammatory pain model. Each model was divided into four groups vehicle, NK1 antagonist, CGRP antagonist, and combination treatment groups. The NK1 antagonist aprepitant (BIBN4096, 1 mg/kg) or the CGRP antagonist olcegepant (MK-0869, 10 mg/kg) was injected intraperitoneally. Mechanical allodynia, thermal hypersensitivity, and anxiety-related behaviors were assessed using the von Frey, hot plate, and elevated plus-maze tests. The flinching and licking responses were also evaluated after formalin injection.
Results
Co-administration of aprepitant and olcegepant more significantly alleviated pain behaviors than administration of single agents or vehicle, increasing the mechanical threshold and improving the response latency. Anxiety-related behaviors were also markedly improved after dual treatment compared with either naive mice or the neuropathic pain model in the dual treatment group. Flinching frequency and licking response after formalin injection decreased significantly in the dual treatment group. Isobolographic analysis showed a meaningful additive effect between the two compounds.
Chronic pain is one of the most costly health problem across the world [1]. Neuropathic pain is one of the leading causes of chronic pain, and it has been estimated to affect 7%–10% of the general population [2]. Gabapentinoids, antidepressants, and even potent opioids have been widely prescribed to treat neuropathic pain [3]. However, many patients do not achieve satisfactory pain relief with evidence-based treatments, such as traditional analgesics, or cannot tolerate these drugs because of their adverse effects [4]. Recent advances in the understanding of the chronic pain have spurred an increased interest in the role of neuropeptides [5]. Substance P (SP) and calcitonin gene-related peptide (CGRP) are the most widely investigated neuropeptides with potential roles in nociception that provide therapeutic targets [6].
Neurokinin-1 (NK1) antagonists for pain therapy were largely based on the concept of interrupting neurotransmission of SP from primary afferent nociceptors to central pain pathways in the spinal dorsal horn and more rostrally [7]. Recent studies of neuropathic pain models have demonstrated that sustained activation of the NK1 receptor maintains pain hypersensitivity [6,8], and that NK1 receptor antagonists inhibit SP-induced activation of spinal neurons and thus prevent pain transmission in preclinical studies [9]. Administration of NK1 receptor antagonists also demonstrated significant antinociceptive effects in the inflammatory pain model [10,11]. Similar to the SP-NK1 receptor system, CGRP plays a role in transmission and modulation of pain signals [12]. Administration of a CGRP receptor antagonist has been reported to prevent the development and maintenance of allodynia/hyperalgesia in rats suffering from neuropathic pain [13,14]. Recent studies show apparent antinociceptive effects by CGRP antagonists in inflammatory pain models [1,12].
Current pharmacological regimens used in pain clinics to treat neuropathic pain frequently include a combination of multiple agents, such as antidepressants, gabapentin, analgesics, and antiepilepsy medications [15,16]. The use of numerous pharmacological treatment strategies involving various medications can increase the success rate of neuropathic pain alleviation and minimize the adverse effects of each drug [15,16]. Agents that block neuropeptide receptors are promising analgesic drug candidates; thus, several studies on the effects of neuropeptide antagonists combined with other pain medicines have been conducted [17–19]. For example, Michot et al. [18] reported that compared with the administration of each drug individually, the combination of naratriptan and a CGRP receptor antagonist more effectively alleviates neuropathic pain caused by chronic constriction injury to the infraorbital nerve in rats. However, few studies have investigated the pain-alleviating effect of the combination of two or more neuropeptide antagonists.
Therefore, we investigated the anti-nociceptive effect of co-administration of the CGRP antagonist and an NK1 antagonist to 1) naive mice, 2) neuropathic pain models, and 3) inflammatory pain models, respectively.
Adult male C57Bl/6J mice aged 8–9 weeks and weighing 25–35 g were used for the mouse experiments. The animals were randomized into the naive mice group, neuropathic pain model group, and inflammatory pain models group. The mice were maintained on a regular 12-hour light/dark cycle and provided food [20]. All animal experiment protocols were approved by the Institutional Animal Care and Use Committee of the Salk Institute for Biological Studies and the Animal Experimentation Committee of Wonkwang University (no. 2020-3791). All efforts were made to minimize animal suffering and reduce the number of animals used.
Neuropathic pain models were induced by a modified unilateral loose sciatic nerve ligation procedure, as described in a previous study [21]. This procedure induces incomplete nerve injury in the animals, as indicated by behavioral signs of allodynia [18]. In detail, the mice were anesthetized by inhalation of 3.5% isoflurane in O2 and anesthesia was maintained by inhalation of 1.5%–2% isoflurane in O2 throughout the surgery. The common sciatic nerve was exposed by blunt dissection at the mid-thigh level. Chromic catgut (5-0) suture was loosely tied four times approximately 1 mm apart along a 5 mm piece of nerve separated from the surrounding tissues [21]. The skin and muscle were closed using silk sutures (4-0). After surgery, the chronic constriction injury model mice were allowed to recover from anesthesia on a warming pad in a postoperative chamber before returning to their home cages.
Mice were treated with the CGRP receptor antagonist olcegepant (BIBN4096, 1 mg/kg; MedChemExpress, Monmouth Junction, NJ) or the NK1 receptor antagonist aprepitant (MK-0869, 10 mg/kg; MedChemExpress) [13,22]. Stock solutions were prepared in 5% dimethyl sulfoxide (DMSO) and administered intraperitoneally 10 once-daily doses over 2 weeks [23]. For combination treatment, the same dose (olcegepant 1 mg/kg and aprepitant 10 mg/kg) was injected into the animal model as in single-drug treatment. The mice were divided into four groups: 1) the control group, which was given vehicle; 2) the olcegepant group; 3) the aprepitant group; and 4) the experimental group, which was given both olcegepant and aprepitant. The drugs were first administered to the neuropathic pain when their responses were stabilized (approximately two weeks after the surgery). Mice showing postsurgical motor symptoms such as weakness or gait abnormalities were excluded from this study.
To determine the antinociceptive effects of the combination of the two neuropeptide antagonists, the von Frey test and hot plate test were performed. Formalin was injected into the right hind paw of the naive mice to investigate the ability of the dual treatment to relieve inflammatory pain. Anxiety-like behaviors associated with pain were analyzed using the Elevated Plus Maze (EPM) test. The naive and pain model mice were subjected to behavioral tests daily before and after the administration of the drugs, and they were subjected to tests 10 days postoperatively when hypersensitivity to mechanical and thermal stimulation had fully developed [18]. Behavioral pain evaluations for neuropathic pain models were started one hour after the drug injection and were tested in order, starting with the EPM, followed by the electronic von Frey test and hot plate test. The formalin test in naive mice was performed the next day after those tests were completed. All behavioral investigation was performed in an observer-blind manner.
To evaluate the response to thermal pain, mice were placed in a cylinder-shaped transparent Plexiglas chamber (11 × 11 × 15 cm) on a hot plate (54°C, PE34; IITC Life Science, Woodland Hills, CA) [20]. The latency to a pain response (shaking the hind paw, licking, or jumping) was measured manually.
To measure the mechanical thresholds of the mice, a Dynamic Plantar Aesthesiometer (37450; Ugo Basile, Gemonio, Italy) was used. Mice were placed in a Plexiglas chamber (10 × 10 × 13 cm) on a metal mesh floor and allowed to habituate for more than 30 minutes [20]. The maximum force of the system was set to 50 g at 20 seconds. A metal rod was placed beneath the hind paw and activated when the mice were immobile but awake [20]. The system automatically measured the latency until and the force delivered when the mice withdrew their hind paw. The test was performed five times at 5–10 minutes intervals, and the average value was used.
To assess inflammatory pain responses, mice were first anesthetized by inhalation of 3.5% isoflurane in O2 and 10 µL of 4% formalin (1.6% paraformaldehyde, 19210; Electron Microscopy Sciences, Hatfield, PA) was injected subcutaneously into right side of the hind paw [20]. The mice were then placed in a Plexiglas chamber (10 × 10 × 13 cm), behind which was a mirror [20]. The pain-related behaviors were quantified by determining the incidence of spontaneous flinching of the injected paw or the cumulative time of licking the injected paw [6]. Nociceptive behaviors were measured every 5 minutes after the injection [24]. Data were recorded for the early acute phase (phase I) observed during 0–5 minutes after the injection and the late tonic phase (phase II) observed during 20–60 minutes after the injection [25].
Since anxiety symptoms and chronic pain have been reported to be closely related [26], the anxiolytic effects of combination treatment were investigated through the EPM test. The EPM apparatus consists of a plus-shaped maze elevated above the floor with two oppositely positioned closed arms, two oppositely positioned open arms, and a center area [27]. As subjects freely explore the maze, their behavior is recorded by means of a video camera mounted above the maze and analyzed using a video tracking system. The preference for being in the open arms over the closed arms (expressed as either as a percentage of entries and/or a percentage of time spent in the open arms) is calculated to measure anxiety-like behavior. To monitor the anxiety-like behaviors, a custom-built EPM with two transparent closed arms (77 × 7 × 30 cm) and two open arms (77 × 7 × 2 cm) was used [20]. This maze was elevated 70 cm above ground for all tests, and the behavior was video recorded for 10 minutes and tracked with video-tracking software (EthoVision XT 12; Noldus, Wageningen, The Netherlands) [20].
To examine the synergistic effects of combination treatment using olcegepant and aprepitant, the isobolographic analysis generated from dose-response data from the individual agents was performed [28]. Mice were divided into a vehicle group (DMSO; n = 6), an olcegepant group (2.5, 5, 10 mg; n = 6), and an aprepitant (50, 100, 200 mg; n = 6) group. After administering each agent, a formalin test was performed to observe the pain response, and the 50% effective dose (ED50) for each drug was calculated using a dose-response curve. The pain inhibition effects (PIE) of the compounds were analyzed using the following formula.
Then, olcegepant and aprepitant of the dose corresponding to the ED50, 50% of the ED50, and 25% of the ED50 were administered in the inflammatory pain model. The isobologram was analyzed using CompuSyn software (ComboSyn Inc., Paramus, NJ) to investigate the interaction of the two drugs.
Time-response data were presented during the observation periods for analyzing the behavioral results. Parametric assumptions were evaluated for all variables using histograms, descriptive statistics, and the Shapiro–Wilk test for normality. After that, a one-way analysis of variance (ANOVA) with Tukey correction was performed for multiple comparisons between groups. A P value less than 0.05 was considered statistically significant. All of the data were statistically analyzed using IBM SPSS 22.0 software (IBM Co., Armonk, NY).
Fifty-six C57BL/6J mice and 56 neuropathic pain model mice were used for this study. Naive mice and neuropathic pain models consist of four groups (vehicle, aprepitant, olcegepant, and combination treatment). Thus, 14 mice were used for each group. Significant mechanical allodynia developed by day five after sciatic nerve ligation in the experimental group, with mechanism allodynia reaching a plateau on day 10 and being maintained until the end of the study. We defined the mice whose mechanical threshold measured by the von Frey test was reduced to less than 0.4 g on the postoperative 10th day after the chronic constriction injury to the neuropathic pain model. These mice were used to investigate the therapeutic effect of neuropeptide antagonists. The mean mechanical threshold of the participating neuropathic pain model was 0.34 ± 0.02 g (n = 56) before the beginning of the behavioral tests, and the naive mice showed 1.22 ± 0.06 g (n = 56).
ANOVA indicated significant effects in the different treatment groups. More specifically, post hoc analysis revealed that compared with the mice in the other groups, naive mice in the dual treatment group showed a significant increase in the mechanical threshold seven days after administration (F2,32 = 21.24, P = 0.048) and that this response was sustained until the 15th day (F2,32 = 32.13, P = 0.036) compared to the other groups (Fig. 1A).
When aprepitant and olcegepant were co-administered to the neuropathic pain model, the resulting increase in the mechanical threshold was much higher. It lasted longer than that induced after treatment with either compound alone (Fig. 1B). There were significant differences in the mechanical threshold between the groups 3 days after administrating aprepitant or olcegepant (F2,32 = 44.36, P = 0.001), and these significant differences were sustained throughout the evaluation period. DMSO-treated mice did not show any significant change in the mechanical threshold.
There was no significant change in thermal hypersensitivity in the naive mice after administrating aprepitant, olcegeant, or both agents (F2,32 = 36.234, P = 0.236) (Fig. 2A). However, compared with the mice in the other groups, the neuropathic pain model showed a significantly increased response latency from the 3rd day of administration (F2,32 = 29.126, P = 0.018). This increase lasted until the last day of the experiment (Fig. 2B). On the other hand, a significant increase in the thermal sensitivity was not observed after the single or vehicle treatments.
Phase I began immediately after injection and peaked during the 5 minutes after a 10–15 minutes quiescent period. Phase II followed and lasted about 60 minutes. During phase I, the total number of formalin-induced flinches was not significantly different among groups (P = 0.623) (Fig. 3A). However, during phase II, significantly less flinching was observed in the dual treatment group. The mice coadministered aprepitant and olcegepant showed significantly less flinching frequency than those with single agent or vehicle administration from 30 minutes (F2,32 = 19.235, P = 0.024) after formalin injection until the end of the experiment. Licking behaviors showed a similar profile to flinching. The cumulative time of the phase I licking response of the naive mice was not significantly different among the groups (Fig. 3B). However, the cumulative licking time was significantly shorter in phase II in the dual treatment group than in the other groups, from 25 minutes to 50 minutes post-injection (F2,32 = 17.058, P = 0.020).
The neuropathic pain model spent less time in the open arms (F2,32 = 15.204, P = 0.001) and more time in the closed arms (F2,32 = 19.112, P = 0.001) of the EPM test setup than the naive mice, regardless of treatment groups. In the naive mice, the coadministration of aprepitant and olcegepant markedly increased the time spent in the open arms (F2,32 = 13.621, P = 0.036, Fig. 4A) while decreasing the time spent in the closed arms (F2,32 = 10.128, P = 0.048, Fig. 4B). The neuropathic pain model in the dual treatment group also stayed significantly longer in the open arms (F2,32 = 13.246, P = 0.005) and spent a shorter time in the closed arm than the mice with a single drug or vehicle injection (F2,32 = 12.194, P = 0.016). After injecting a single agent, either aprepitant or olcegepant, the time the naive mice or neuropathic pain model remained in the open or closed arms was not significantly different. The total arm entries in the EPM tests were comparable among the investigated groups of mice, indicating that regular locomotor activity is maintained after aprepitant or olcegepant injection (Fig. 4C). Both naive mice and neuropathic pain models treated with vehicles did not show any significant anxiolytic effects.
The dose-response effect of each compound and combination treatment were shown in Fig. 5. The ED50 obtained by the dose-response curve was 5.8 mg (4.6–7.1) in olcegepant and 104.3 mg (94.4–114.5) in aprepitant. In addition, the ED50 of combination treatment measured by the dose-response curve was 2.5 mg (2.1–2.9) in olcegepant and 46.6 mg (41.6–51.6) in aprepitant. Since the experimental value of the ED50 of combination treatment was located lower-left than the theoretical ED50, isobologram showed a meaningful additive effect between the two drugs (Fig. 6).
In this study, combination treatment with aprepitant and olcegepant had better mitigated neuropathic and inflammatory pain than a single neuropeptide antagonist. In addition to exerting an antinociceptive effect, the combination of the drugs reduced anxiety due to pain greater than administration of a single drug. These findings suggest that combination treatment strategies comprising multiple neuropeptide antagonists are required to alleviate neuropathic and inflammatory pain effectively.
Dual treatment with the NK1 receptor antagonist aprepitant and the CGRP receptor antagonist olcegepant reduced pain sensitivity in either neuropathic pain model or naive mice. There were no significant changes in pain behavior indicators evaluated by the von Frey test or hot plate test in the group where single drug was administered. Although agents that block neuropeptide receptors have been identified as promising analgesic drug candidates based on studies in animal models, they have failed in several clinical trials [29–31]. It is unclear why the trials failed, but multiple neuropeptides which generate and transmit neuropathic pain might be a crucial cause of these failures [6,32,33]. Our results are similar to those of previous studies showing that the combination of neuropeptide antagonists and traditional pain killers such as opioids or nonsteroidal anti-inflammatory drugs has a better antinociceptive effect than treatment with a single drug [17,18]. In particular, it is necessary to consider combination drug strategies to treat neuropathic pain since this condition is caused by various complex mechanisms involving diverse neuropeptides.
Dual pharmacological treatment with aprepitant and olcegepant had more significant anxiolytic effects in both neuropathic pain model and naive mice than treatment with a single agent, as evaluated by the EPM test. Anxiety is reported in up to 45% of patients with chronic pain [34], and the relationship between pain and anxiety has been widely studied in clinical and preclinical studies [35–38]. High levels of SP and its NK1 receptor have also been detected throughout the whole rat brain, including areas related to anxiety [39]. Several studies have assessed the anti-anxiety effect of NK1 antagonists in animal models [40,41]. For example, Borbély et al. [41] reported that a high dose of the NK1 antagonist CP99994 significantly attenuates anxiety-like behavior in Tac1-/- mice. Similar to SP, CGRP contributes to anxiety responses caused by pain. An increase in CGRP levels induces anxiety-like behaviors in animal models, and administration of the CGRP antagonist CGRP8–37 or BIBN4096BS significantly attenuates these behaviors in C57BL/6 mice [42,43]. Our findings are consistent with those of previous studies, and dual therapy may be more effective in relieving comorbidities caused by pain, such as anxiety.
Intraperitoneal injection of both aprepitant and olcegepant more effectively attenuated formalin-induced nociceptive behaviors in phase II than administration of a single agent. The reason that the mice, after formalin injection, showed anti-nociceptive behavior in phase II only was assumed to be due to the different mechanisms of the two phases. The response in phase I is caused by the direct activation of thin centrifugal nerve fibers (Aδ and C fibers) distributed in the damaged tissue, and peripheral and central sensitization resulting from an inflammatory response caused by repetitive stimulation of C fibers induces the phase II response [44,45]. The previous studies reported that the neuropeptide antagonists such as NK1 or CGRP eliminated latent sensitization induced by inflammation or nerve injury [5,8]. Latent sensitization of the dorsal horn is known as a crucial component of the chronic pain circuitry on the supraspinal level in rodents [8]. Allodynia induced by stress in rats was improved after blocking latent sensitization by administrating NK1 antagonist to block latent sensitization [46]. The latent sensitization showed that the chronic pain state is radically different from regular pain transmission. Our results suggested that co-administration of neuropeptide antagonists could be more effectively used for alleviating chronic or affective pain in clinical practice.
There are several limitations to this study. We injected aprepitant and olcegepant into the mice at the doses recommended by the pharmaceutical companies. Antinociceptive medicines, including neuropeptide antagonists, generally exert a dose-dependent effect in alleviating pain. Additional experiments are required to determine the optimal dosage of these neuropeptide antagonists. We observed changes in pain and anxiety-like behavior after dual neuropeptide antagonist therapy. However, the regulation of NK1 and CGRP receptor activity and changes in neuronal activity in tissues were not evaluated. Further studies are required to overcome the limitations of this study.
In summary, combination treatment with multiple neuropeptide antagonists exerted better therapeutic effects than the conventional single-agent regimen in the pain model. More marked amelioration of anxiety-like behaviors comorbid with pain was observed simultaneously after treatment with aprepitant and olcegepant. A combination pharmacological therapy comprising multiple neuropeptide antagonists could be a more effective therapeutic strategy for alleviating neuropathic or inflammatory pain.
Notes
REFERENCES
1. Schou WS, Ashina S, Amin FM, Goadsby PJ, Ashina M. 2017; Calcitonin gene-related peptide and pain: a systematic review. J Headache Pain. 18:34. DOI: 10.1186/s10194-017-0741-2. PMID: 28303458. PMCID: PMC5355411.

2. van Hecke O, Austin SK, Khan RA, Smith BH, Torrance N. 2014; Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain. 155:654–62. DOI: 10.1016/j.pain.2013.11.013. PMID: 24291734.

3. Cavalli E, Mammana S, Nicoletti F, Bramanti P, Mazzon E. 2019; The neuropathic pain: an overview of the current treatment and future therapeutic approaches. Int J Immunopathol Pharmacol. 33:2058738419838383. DOI: 10.1177/2058738419838383. PMID: 30900486. PMCID: PMC6431761.

4. Bouhassira D, Chassany O, Gaillat J, Hanslik T, Launay O, Mann C, et al. 2012; Patient perspective on herpes zoster and its complications: an observational prospective study in patients aged over 50 years in general practice. Pain. 153:342–9. DOI: 10.1016/j.pain.2011.10.026. PMID: 22138256.

5. Iyengar S, Ossipov MH, Johnson KW. 2017; The role of calcitonin gene-related peptide in peripheral and central pain mechanisms including migraine. Pain. 158:543–59. DOI: 10.1097/j.pain.0000000000000831. PMID: 28301400. PMCID: PMC5359791.

6. Hunyady Á, Hajna Z, Gubányi T, Scheich B, Kemény Á, Gaszner B, et al. 2019; Hemokinin-1 is an important mediator of pain in mouse models of neuropathic and inflammatory mechanisms. Brain Res Bull. 147:165–73. DOI: 10.1016/j.brainresbull.2019.01.015. PMID: 30664920.

7. Navratilova E, Porreca F. 2019; Substance P and inflammatory pain: getting it wrong and right simultaneously. Neuron. 101:353–5. DOI: 10.1016/j.neuron.2019.01.034. PMID: 30731054.

8. Chen W, Marvizon JC. 2020; Neurokinin 1 receptor activation in the rat spinal cord maintains latent sensitization, a model of inflammatory and neuropathic chronic pain. Neuropharmacology. 177:108253. DOI: 10.1016/j.neuropharm.2020.108253. PMID: 32736088.

9. Ramírez-García PD, Retamal JS, Shenoy P, Imlach W, Sykes M, Truong N, et al. 2019; A pH-responsive nanoparticle targets the neurokinin 1 receptor in endosomes to prevent chronic pain. Nat Nanotechnol. 14:1150–9. DOI: 10.1038/s41565-019-0568-x. PMID: 31686009. PMCID: PMC7765343.

10. Teodoro FC, Tronco Júnior MF, Zampronio AR, Martini AC, Rae GA, Chichorro JG. 2013; Peripheral substance P and neurokinin-1 receptors have a role in inflammatory and neuropathic orofacial pain models. Neuropeptides. 47:199–206. DOI: 10.1016/j.npep.2012.10.005. PMID: 23177733.

11. Gautam M, Prasoon P, Kumar R, Reeta KH, Kaler S, Ray SB. 2016; Role of neurokinin type 1 receptor in nociception at the periphery and the spinal level in the rat. Spinal Cord. 54:172–82. DOI: 10.1038/sc.2015.206. PMID: 26690860.

12. Hirsch S, Corradini L, Just S, Arndt K, Doods H. 2013; The CGRP receptor antagonist BIBN4096BS peripherally alleviates inflammatory pain in rats. Pain. 154:700–7. DOI: 10.1016/j.pain.2013.01.002. PMID: 23473785.

13. Christensen SL, Petersen S, Kristensen DM, Olesen J, Munro G. 2019; Targeting CGRP via receptor antagonism and antibody neutralisation in two distinct rodent models of migraine-like pain. Cephalalgia. 39:1827–37. DOI: 10.1177/0333102419861726. PMID: 31288556.

14. Lee SE, Kim JH. 2007; Involvement of substance P and calcitonin gene-related peptide in development and maintenance of neuropathic pain from spinal nerve injury model of rat. Neurosci Res. 58:245–9. DOI: 10.1016/j.neures.2007.03.004. PMID: 17428562.

15. Baron R, Binder A, Wasner G. 2010; Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 9:807–19. DOI: 10.1016/S1474-4422(10)70143-5.

16. Finnerup NB, Kuner R, Jensen TS. 2021; Neuropathic pain: from mechanisms to treatment. Physiol Rev. 101:259–301. DOI: 10.1152/physrev.00045.2019. PMID: 32584191.

17. Hama A, Sagen J. 2014; Selective antinociceptive effects of a combination of the N-methyl-D-aspartate receptor peptide antagonist [Ser(1)]histogranin and morphine in rat models of pain. Pharmacol Res Perspect. 2:e00032. DOI: 10.1002/prp2.32. PMID: 25505581. PMCID: PMC4184704.

18. Michot B, Bourgoin S, Viguier F, Hamon M, Kayser V. 2012; Differential effects of calcitonin gene-related peptide receptor blockade by olcegepant on mechanical allodynia induced by ligation of the infraorbital nerve vs the sciatic nerve in the rat. Pain. 153:1939–48. DOI: 10.1016/j.pain.2012.06.009. PMID: 22795918.

19. Betti C, Starnowska J, Mika J, Dyniewicz J, Frankiewicz L, Novoa A, et al. 2015; dual alleviation of acute and neuropathic pain by fused opioid agonist-neurokinin 1 antagonist peptidomimetics. ACS Med Chem Lett. 6:1209–14. DOI: 10.1021/acsmedchemlett.5b00359. PMID: 26713106. PMCID: PMC4677362.

20. Kang SJ, Liu S, Ye M, Kim DI, Kim JH, Oh TG, et al. 2020. Unified neural pathways that gate affective pain and multisensory innate threat signals to the amygdala. BioRxiv. 2020.11.17.385104 [Preprint]. Available from: https://doi.org/10.1101/2020.11.17.385104. cited 2020 Nov 18. DOI: 10.1101/2020.11.17.385104.

21. Medeiros P, Dos Santos IR, Júnior IM, Palazzo E, da Silva JA, Machado HR, et al. 2021; An adapted chronic constriction injury of the sciatic nerve produces sensory, affective, and cognitive impairments: a peripheral mononeuropathy model for the study of comorbid neuropsychiatric disorders associated with neuropathic pain in rats. Pain Med. 22:338–51. DOI: 10.1093/pm/pnaa206. PMID: 32875331.

22. Mannangatti P, Sundaramurthy S, Ramamoorthy S, Jayanthi LD. 2017; Differential effects of aprepitant, a clinically used neurokinin-1 receptor antagonist on the expression of conditioned psychostimulant versus opioid reward. Psychopharmacology (Berl). 234:695–705. DOI: 10.1007/s00213-016-4504-6. PMID: 28013351. PMCID: PMC5266628.

23. Mulder IA, Li M, de Vries T, Qin T, Yanagisawa T, Sugimoto K, et al. 2020; Anti-migraine calcitonin gene-related peptide receptor antagonists worsen cerebral ischemic outcome in mice. Ann Neurol. 88:771–84. DOI: 10.1002/ana.25831. PMID: 32583883. PMCID: PMC7540520.

24. Lee IO, Jeong YS. 2002; Effects of different concentrations of formalin on paw edema and pain behaviors in rats. J Korean Med Sci. 17:81–5. DOI: 10.3346/jkms.2002.17.1.81. PMID: 11850594. PMCID: PMC3054816.

25. Greco R, Mangione AS, Siani F, Blandini F, Vairetti M, Nappi G, et al. 2014; Effects of CGRP receptor antagonism in nitroglycerin-induced hyperalgesia. Cephalalgia. 34:594–604. DOI: 10.1177/0333102413517776. PMID: 24366981.

26. Zhuo M. 2016; Neural mechanisms underlying anxiety-chronic pain interactions. Trends Neurosci. 39:136–45. DOI: 10.1016/j.tins.2016.01.006. PMID: 26878750.

27. Walf AA, Frye CA. 2007; The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2:322–8. DOI: 10.1038/nprot.2007.44. PMID: 17406592. PMCID: PMC3623971.

28. Tallarida RJ. 2016; Drug combinations: tests and analysis with isoboles. Curr Protoc Pharmacol. 72:9.19.1–19. DOI: 10.1002/0471141755.ph0919s72. PMID: 26995550. PMCID: PMC4839184.

29. Keller M, Montgomery S, Ball W, Morrison M, Snavely D, Liu G, et al. 2006; Lack of efficacy of the substance p (neurokinin1 receptor) antagonist aprepitant in the treatment of major depressive disorder. Biol Psychiatry. 59:216–23. DOI: 10.1016/j.biopsych.2005.07.013. PMID: 16248986.

30. Tebas P, Tuluc F, Barrett JS, Wagner W, Kim D, Zhao H, et al. 2011; A randomized, placebo controlled, double masked phase IB study evaluating the safety and antiviral activity of aprepitant, a neurokinin-1 receptor antagonist in HIV-1 infected adults. PLoS One. 6:e24180. DOI: 10.1371/journal.pone.0024180. PMID: 21931661. PMCID: PMC3169584.

31. Voss T, Lipton RB, Dodick DW, Dupre N, Ge JY, Bachman R, et al. 2016; A phase IIb randomized, double-blind, placebo-controlled trial of ubrogepant for the acute treatment of migraine. Cephalalgia. 36:887–98. DOI: 10.1177/0333102416653233. PMID: 27269043.

32. Herbert MK, Holzer P. 2002; [Why are substance P(NK1)-receptor antagonists ineffective in pain treatment?]. Anaesthesist. 51:308–19. German. DOI: 10.1007/s00101-002-0296-7. PMID: 12063723.
33. Hill R. 2000; NK1 (substance P) receptor antagonists--why are they not analgesic in humans? Trends Pharmacol Sci. 21:244–6. DOI: 10.1016/S0165-6147(00)01502-9.
34. Kroenke K, Outcalt S, Krebs E, Bair MJ, Wu J, Chumbler N, et al. 2013; Association between anxiety, health-related quality of life and functional impairment in primary care patients with chronic pain. Gen Hosp Psychiatry. 35:359–65. DOI: 10.1016/j.genhosppsych.2013.03.020. PMID: 23639186.

35. De Gregorio D, McLaughlin RJ, Posa L, Ochoa-Sanchez R, Enns J, Lopez-Canul M, et al. 2019; Cannabidiol modulates serotonergic transmission and reverses both allodynia and anxiety-like behavior in a model of neuropathic pain. Pain. 160:136–50. DOI: 10.1097/j.pain.0000000000001386. PMID: 30157131. PMCID: PMC6319597.

36. McWilliams LA, Goodwin RD, Cox BJ. 2004; Depression and anxiety associated with three pain conditions: results from a nationally representative sample. Pain. 111:77–83. DOI: 10.1016/j.pain.2004.06.002. PMID: 15327811.

37. Roeska K, Doods H, Arndt K, Treede RD, Ceci A. 2008; Anxiety-like behaviour in rats with mononeuropathy is reduced by the analgesic drugs morphine and gabapentin. Pain. 139:349–57. DOI: 10.1016/j.pain.2008.05.003. PMID: 18565660.

38. Wu Y, Yao X, Jiang Y, He X, Shao X, Du J, et al. 2017; Pain aversion and anxiety-like behavior occur at different times during the course of chronic inflammatory pain in rats. J Pain Res. 10:2585–93. DOI: 10.2147/JPR.S139679. PMID: 29158690. PMCID: PMC5683785.

39. Muñoz M, Coveñas R. 2014; Involvement of substance P and the NK-1 receptor in human pathology. Amino Acids. 46:1727–50. DOI: 10.1007/s00726-014-1736-9. PMID: 24705689.

40. Zocchi A, Varnier G, Arban R, Griffante C, Zanetti L, Bettelini L, et al. 2003; Effects of antidepressant drugs and GR 205171, an neurokinin-1 (NK1) receptor antagonist, on the response in the forced swim test and on monoamine extracellular levels in the frontal cortex of the mouse. Neurosci Lett. 345:73–6. DOI: 10.1016/S0304-3940(03)00305-7.

41. Borbély É, Hajna Z, Nabi L, Scheich B, Tékus V, László K, et al. 2017; Hemokinin-1 mediates anxiolytic and anti-depressant-like actions in mice. Brain Behav Immun. 59:219–32. DOI: 10.1016/j.bbi.2016.09.004. PMID: 27621226.

42. Schorscher-Petcu A, Austin JS, Mogil JS, Quirion R. 2009; Role of central calcitonin gene-related peptide (CGRP) in locomotor and anxiety- and depression-like behaviors in two mouse strains exhibiting a CGRP-dependent difference in thermal pain sensitivity. J Mol Neurosci. 39:125–36. DOI: 10.1007/s12031-009-9201-z. PMID: 19381879.

43. Araya EI, Turnes JM, Barroso AR, Chichorro JG. 2020; Contribution of intraganglionic CGRP to migraine-like responses in male and female rats. Cephalalgia. 40:689–700. DOI: 10.1177/0333102419896539. PMID: 31856582.

44. Puig S, Sorkin LS. 1996; Formalin-evoked activity in identified primary afferent fibers: systemic lidocaine suppresses phase-2 activity. Pain. 64:345–55. DOI: 10.1016/0304-3959(95)00121-2.

45. Cho SY, Park AR, Yoon MH, Lee HG, Kim WM, Choi JI. 2013; Antinociceptive effect of intrathecal nefopam and interaction with morphine in formalin-induced pain of rats. Korean J Pain. 26:14–20. DOI: 10.3344/kjp.2013.26.1.14. PMID: 23342202. PMCID: PMC3546204.

46. Rivat C, Laboureyras E, Laulin JP, Le Roy C, Richebé P, Simonnet G. 2007; Non-nociceptive environmental stress induces hyperalgesia, not analgesia, in pain and opioid-experienced rats. Neuropsychopharmacology. 32:2217–28. DOI: 10.1038/sj.npp.1301340. PMID: 17299508.

Fig. 1
Change of mechanical threshold measured with von Frey test after administrating aprepitant (APR) and olcegepant (OLC). (A) The mechanical threshold in naive mice increased significantly with co-administration of both drugs intraperitoneally. (B) More remarkable improvement of mechanical threshold was observed in the dual-treated neuropathic pain model compared to single-treated groups or vehicle. The error bars indicate standard deviation. *P < 0.05 compared with vehicle.
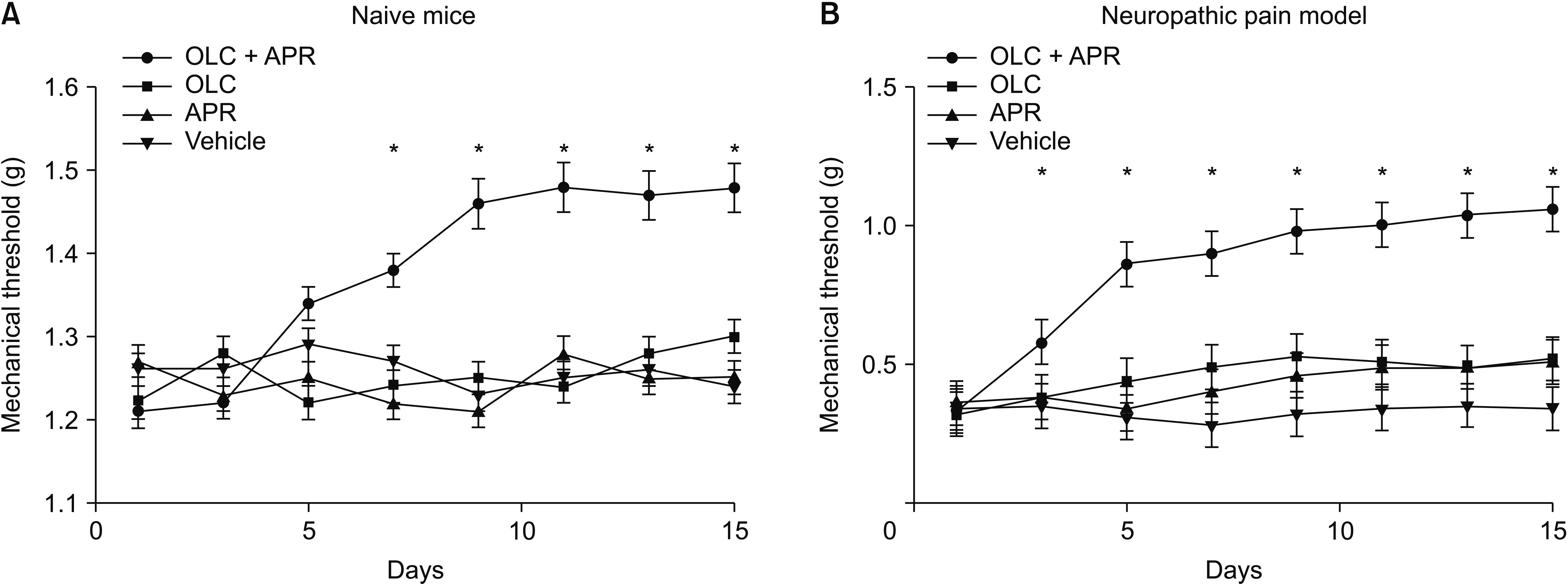
Fig. 2
Effects on the thermal hypersensitivity. (A) Combination treatment by aprepitant (APR) and olcegepant (OLC) significantly attenuated the thermal hypersensitivity over time in naive mice. (B) There was a significant increase in response latency for thermal hypersensitivity in the neuropathic pain model in dual treatment groups. There were no changes in response latency in either a single treatment group or vehicle. The error bars indicate standard deviation. *P < 0.05 compared with vehicle.
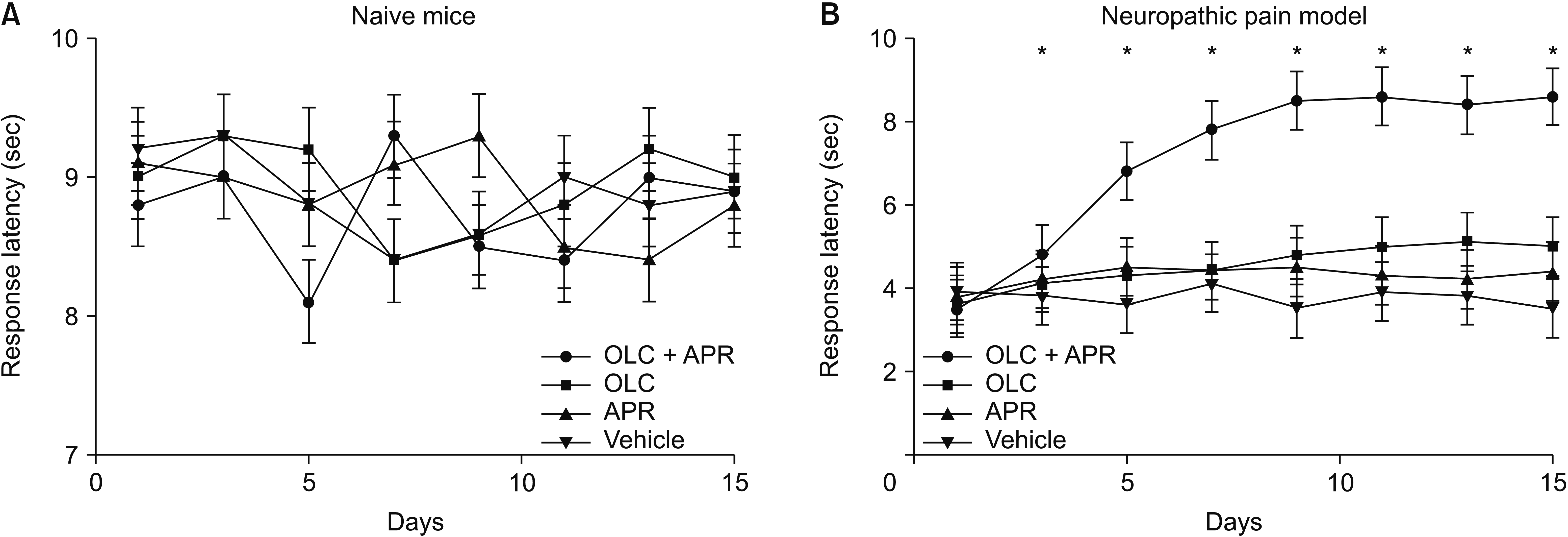
Fig. 3
Effects on formalin-induced inflammatory pain. (A) The number of flinching significantly decreased in the dual treatment group from 30 minutes after formalin injection compared to either single or vehicle administration. (B) Licking cumulative time in the dual treatment group was significantly shorter than in other groups in phase II. APR: aprepitant, OLC: olcegepant. The error bars indicate standard deviation. *P < 0.05 compared with vehicle.
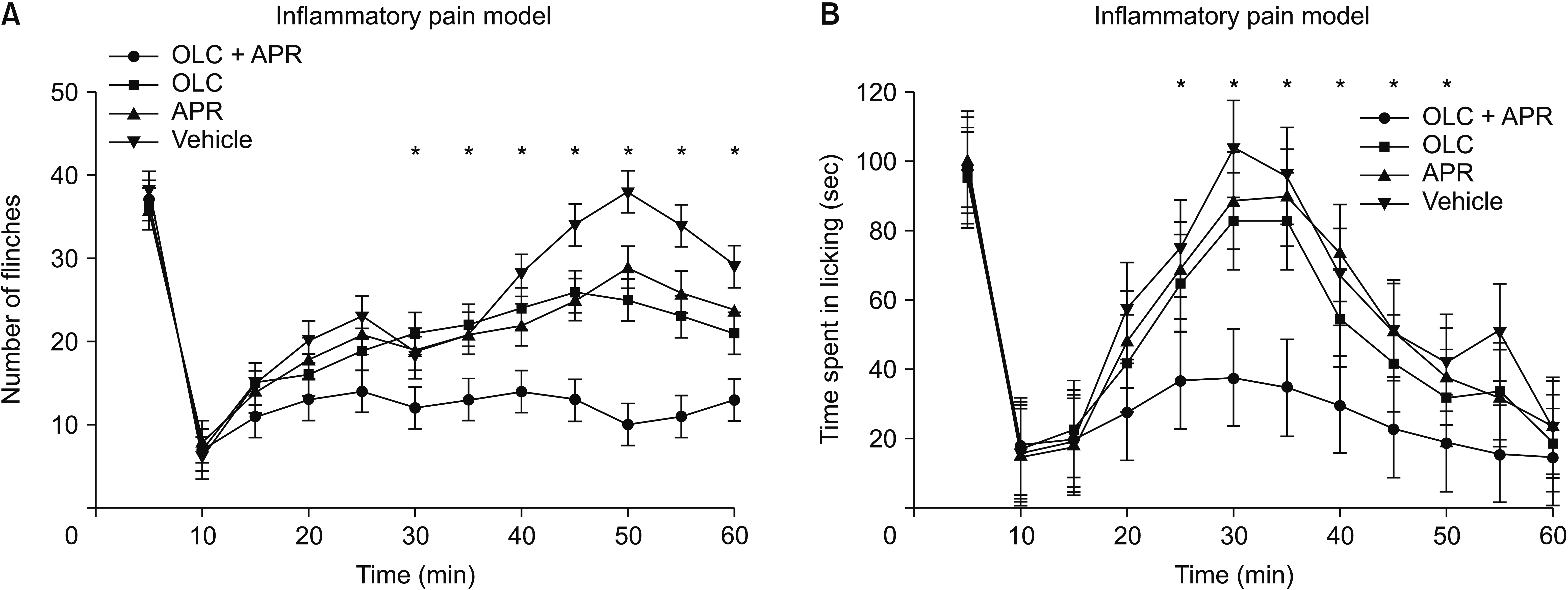
Fig. 4
Effect of co-administration on anxiety-like behavior measured by the elevated plus-maze test. (A) In both naive mice and neuropathic pain models, the co-administration of aprepitant (APR) and olcegepant (OLC) markedly increased the time spent in the open arms compared with the mice treated by single compound or vehicle. (B) In the dual treatment group, naive mice or neuropathic pain model significantly spent less time than the single compound or vehicle treated mice in the close arms. (C) The total arm entries didn’t show significant differences among groups, either naive mice or neuropathic pain model. The error bars indicate standard deviation. *P < 0.05 compared with vehicle or single compound groups.
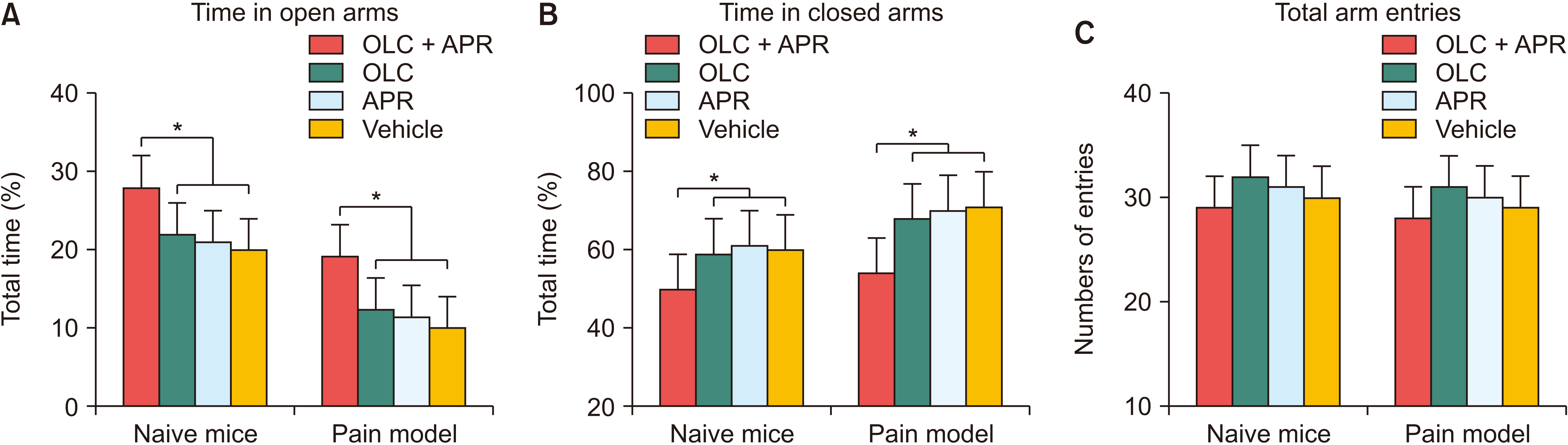
Fig. 5
Dose-response effects of the compounds analyzed by the flinching number after formalin injection. Data are presented as the percent of pain inhibition effect (% PIE). (A) Olcegepant (OLC). (B) Aprepitant (APR). (C) Combination treatment. The error bars indicate standard deviation. *P < 0.05 compared with vehicle.
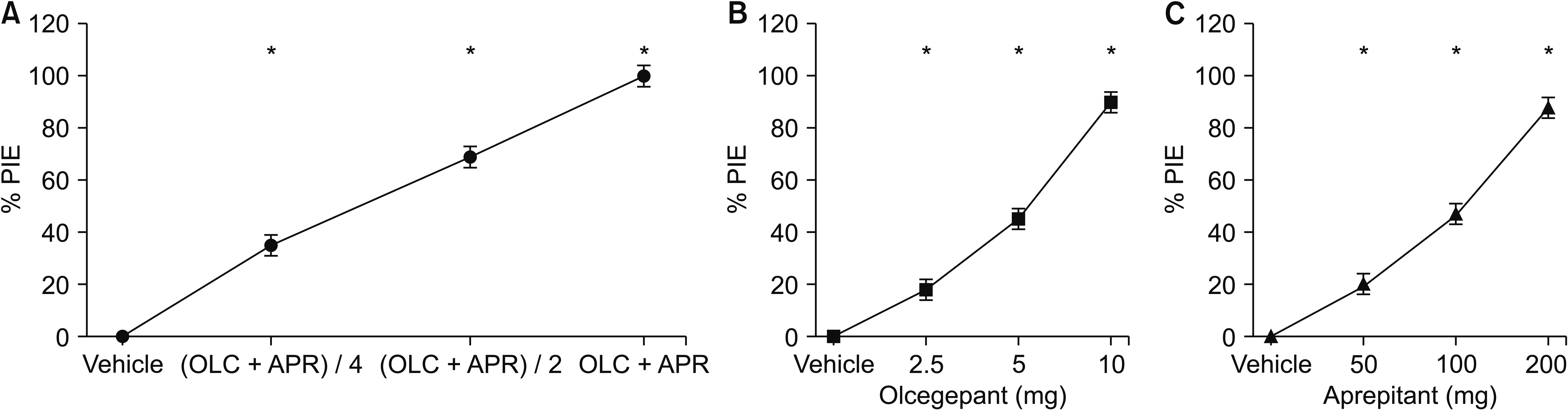
Fig. 6
Isobologram for the interaction of olcegepant and aprepitant. The diagonal line connecting two 50% effective dose (ED50) points is the theoretical line of additivity. The experimental ED50 point was decreased significantly below the line of additivity, indicating a meaningful additive effect.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download