INTRODUCTION
MATERIALS AND METHODS
1. Primary neurons culture
2. Hyperglycemia culture
3. Insulin treatment
4. Immunocytochemistry
5. Live and dead assay
6. SCs proliferation assay
7. Image acquisition and analysis
8. Statistical analyses
 | Fig. 1Schwann cell proliferation is attenuated in culture under hyperglycemia. (A) Representative images show changes in cell morphology and confluence under hyperglycemic culture. Scale bar: 300 µm. (B) Representative images of live and dead assay performed at 7 days of culture in control and hyperglycemic conditions. Scale bar: 100 µm. (C) The graph shows the number of cells at 1, 3, 5, and 7 days of culture in control medium (5.5 mM glucose) and in hyperglycemic medium containing 10, 30, and 60 mM glucose. At 1 day, n = 4 wells each group; non-repeated-measures ANOVA, not significant. At 3 days, n = 4 wells each group; non-repeated-measures ANOVA followed by Dunnett’s post hoc test, F (3, 12) = 6.327, **P < 0.01 vs. control group. At 5 days, n = 6 wells each group; non-repeated-measures ANOVA followed by Dunnett’s post hoc test, F (3, 20) = 16.09, *P < 0.05, **P < 0.01, and ****P < 0.0001 vs. control group. At 7 days, n = 7 wells each group; non-repeated-measures ANOVA followed by Dunnett’s post hoc test, F (3, 24) = 24.47, ****P < 0.0001 vs. control group. (D) The graph shows the percentage of dead cells at 7 days of culture in control medium (5.5 mM glucose) and in hyperglycemic media. n = 5 wells each group; non-repeated-measures ANOVA followed by Dunnett’s post hoc test, F (3, 16) = 43.92, *P < 0.05 and ****P < 0.0001 vs. control group. |
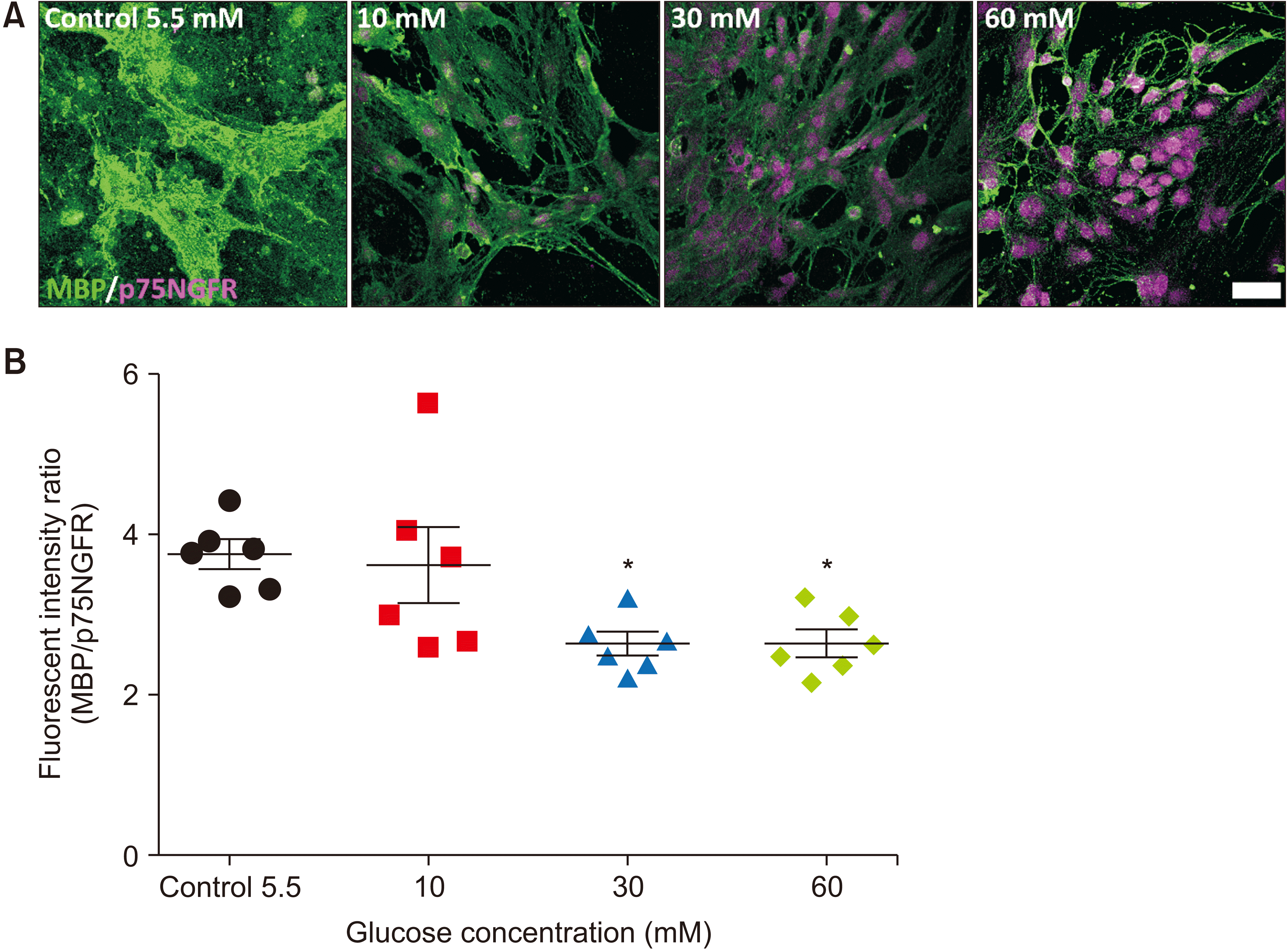 | Fig. 2Schwann cell maturation is impaired under hyperglycemia. (A) Representative images of immunocytochemistry with p75NGFR and MBP performed at 7 days of culture in control and hyperglycemic conditions. Scale bar: 50 µm. (B) The graph shows the fluorescent intensity ratio of MBP and p75NGFR at 7 days of culture in control medium (5.5 mM glucose) and in hyperglycemic medium containing 10, 30, and 60 mM glucose. n = 6, *P < 0.05 vs. control group. n = 6; non-repeated-measures ANOVA followed by Dunnett’s post hoc test, F (3, 20) = 4.845, *P < 0.05 vs. control group. |
 | Fig. 3Hyperglycemia impairs neurite extension. (A, B) Representative images of sensory (A) and motor (B) neurons stained for NF-160 at 2 days of culture in control and hyperglycemic conditions. Scale bar: 50 µm. (C, D) The graph shows the significantly decreased average neurite length of sensory (C) and motor (D) neurons under hyperglycemia at 2 days of culture. (C) n = 3 wells each group, non-repeated-measures ANOVA followed by Dunnett’s post hoc test, F (3, 8) = 32.57, ***P < 0.001 vs. control group. (D) n = 3 wells each group, non-repeated-measures ANOVA followed by Dunnett’s post hoc test, F (3, 8) = 22.14, **P < 0.01 and ***P < 0.001 vs. control group. |
 | Fig. 4Hyperglycemia impairs myelination of sensory neurites. (A) Representative images of sensory neurons stained for NF-160 and MBP at 7 days of co-culture with Schwann cells in control and hyperglycemic conditions. Scale bar: 100 µm. (B) The graph shows the significantly decreased percentage of myelinated sensory neurites under hyperglycemia. n = 3 wells each group; non-repeated-measures ANOVA followed by Dunnett’s post hoc test, F (3, 8) = 50.10, **P < 0.01 and ****P < 0.0001 vs. control group. |
 | Fig. 5Hyperglycemia impairs myelination of motor neurites. (A) Representative images of motor neurons stained for NF-160 and MBP at 7 days of co-culture with Schwann cells in control and hyperglycemic conditions. Scale bar: 100 µm. (B) The graph shows the significantly decreased percentage of myelinated motor neurites under hyperglycemia. n = 3 wells each group; non-repeated-measures ANOVA followed by Dunnett’s post hoc test, F (3, 8) = 238.5, ****P < 0.0001 vs. control group. |
 | Fig. 6Insulin promotes neurite extension. (A, B) Representative images of sensory (A) and motor (B) neurons stained for NF-160 at 2 days of culture in neurobasal medium (25 mM glucose) supplemented with a series of insulin concentrations ranging from 0.01–2 µM. Scale bar: 50 µm. (C) The graph shows the significantly different average neurite length of sensory neurons under the effect of insulin. n = 3 wells each group; non-repeated-measures ANOVA followed by Tukey’s post hoc test, F (6, 14) = 3.970, *P < 0.05 vs. control group. (D) The graph shows the significantly different average neurite length of motor neurons under the effect of insulin. n = 3 wells each group; non-repeated-measures ANOVA followed by Tukey’s post hoc test, F (6, 14) = 47.26, ****P < 0.0001 vs. control group; #P < 0.05 and ##P < 0.01 vs. 0.01 µM; ιιιP < 0.001 and ιιιιP < 0.0001 vs. 0.05 µM; aP < 0.0001 vs. 0.1 µM. |
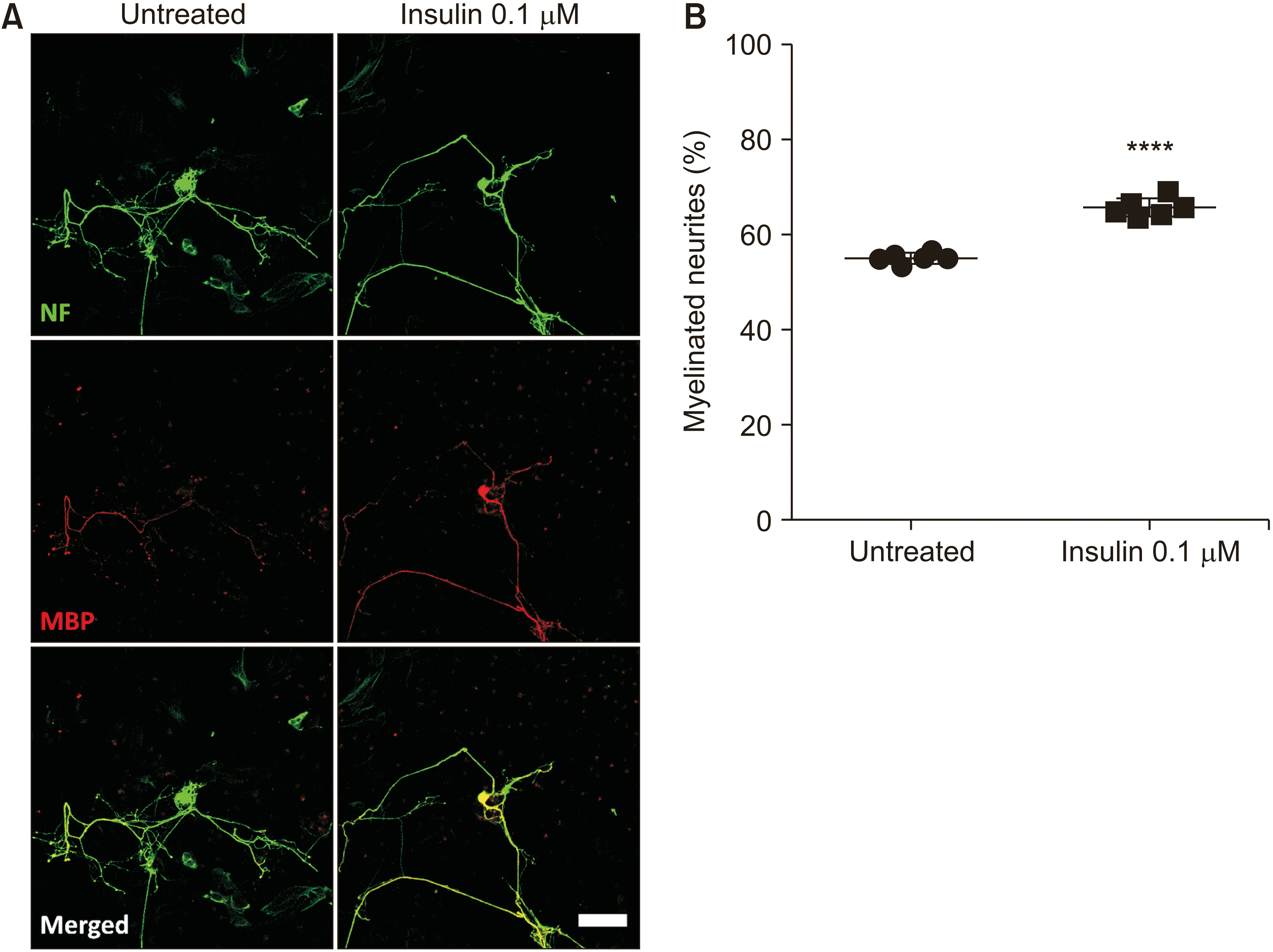 | Fig. 7Insulin promotes sensory neurite myelination. (A) Representative images of sensory neurons stained for NF-160 and MBP at 7 days of co-culture with Schwann cells in hyperglycemic insult (60 mM glucose) supplemented with insulin at 0.1 µM. Scale bar: 100 µm. (B) The graph shows the significantly promoted percentage of myelinated sensory neurites in the group treated by insulin. n = 6; two-tailed Student’s t-test, t (4) = 8.135, ****P < 0.0001. |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download