INTRODUCTION
MATERIALS AND METHODS
1. Experimental animals
2. Plant material
3. Raw materials preparation
4. Essential oil extraction
5. Determination of essential oil chemical composition
Table 1
6. Chemicals, compounds, and drugs
7. The formalin test
8. The hot plate test
9. The investigation of cytotoxicity and growth inhibition
10. Statistical analysis
RESULTS
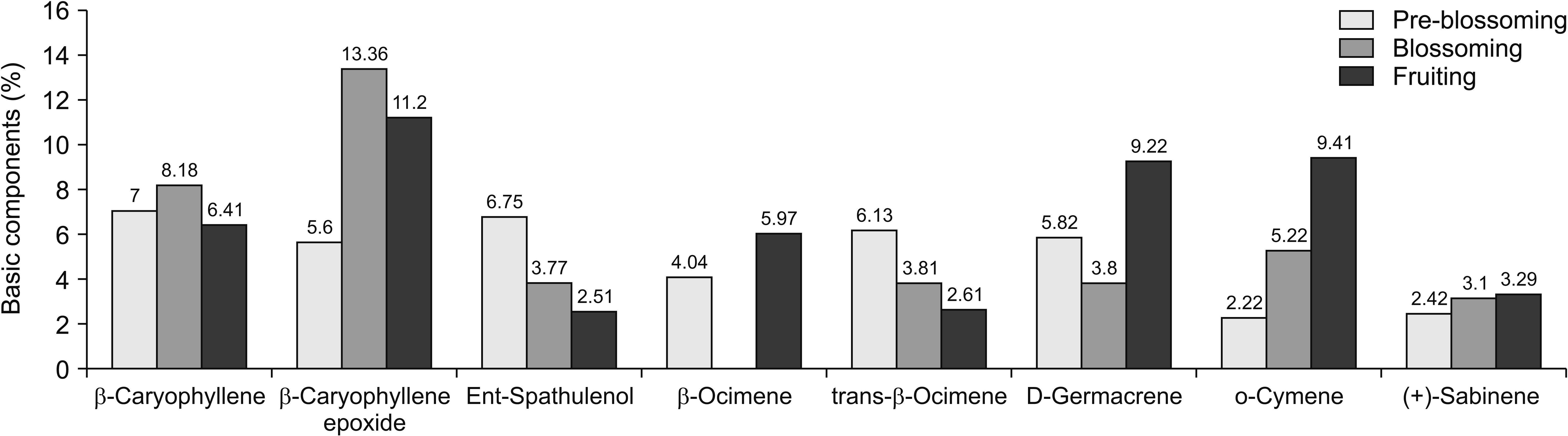
1. Chemical composition of the OVA essential oil in different periods of vegetation: GC-MS analysis
2. Pre-blossoming
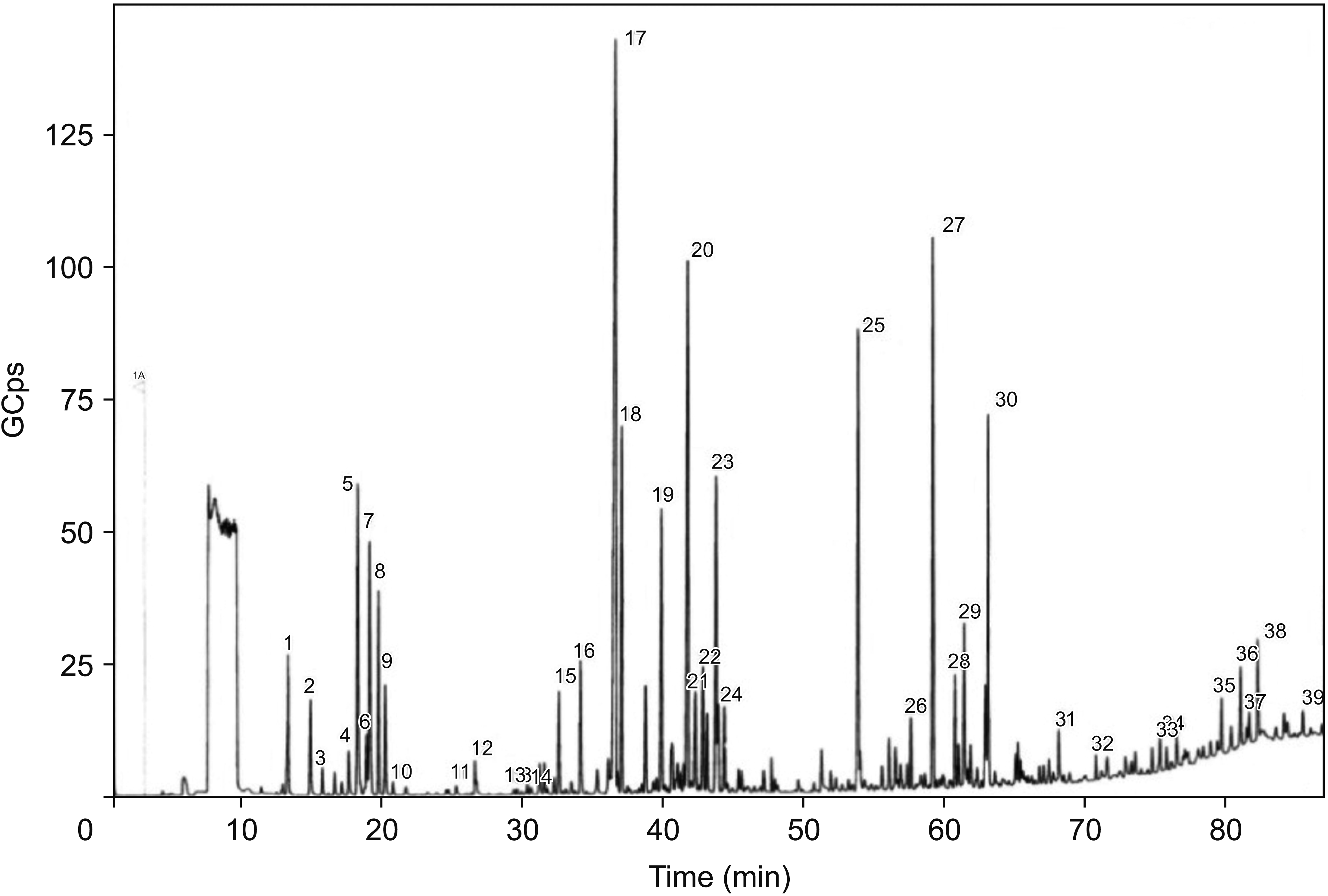 | Fig. 1GC-MS chromotograph of OVA essential oil in the period of pre-blossoming. 1: (+)-Sabinene, 2: β-Pinene, 3: α-Terpinolene, 4: Eucalyptol, 5: trans-β-Ocimene, 6: 1-Isopropyl-4-methyl-1,4-cyclohexadiene, 7: β-Ocimene, 8: Ethyl amyl ketone, 9: ο-Cymene, 10: trans-β-Terpineol, 11: Neo-allo-ocimene, 12: 3-Octanol, 13: p-Menthan-3-one, 14: Elixene, 15: (-)-β-Bourbonene, 16: β-Linalool, 17: β-Caryophyllene, 18: L-4-terpineol, 19: α-Humulen, 20: D-Germacrene, 21: α-Muurolene, 22: γ-Elemene, 23: 1-Isopropyl-4,7-dimethyl-1,2,3,5,6,8a-hexahydronaphtalene, 24: (R)-(+)-β-Citronellol, 25: β-Caryophyllene epoxide, 26: d-Viridiflorol, 27: Ent-Spathulenol, 28: 1-Methyl-4-(methylethyl)-(E)-2-cyclohexenol, 29: tau-Cadinol, 30: α-Cadinol, 31: 6-Isopropyl-4,8a-dimethyl-1,2,3,5,6,7,8,8a-octahydro-2-naphthalenol, 32: Methyl 1,5,5-trimethyl-6-[(1E)-3-methyl-1,3-butadienyl]-7-oxabicyclo[4.1.0.]hept-2-yl ether, 33: trans-Phytol, 34: Diepicadrene-1-oxide, 35: (3E)-4-(1,5-Dihydroxy-2,6,6-trimethyl-2-cyclohexen-1-yl)-3-buten-2-one, 36: cis-Z-α-Bisabolene epoxide, 37: 3-[(2E)-2-Dodecenyl]dihydro-2,5-furandione, 38: Isoaromadendrene epoxide, 39: 2-Methyl-4-(2,6,6-trimethylcyclohex-1-enyl)but-2-en-1-ol. GC-MS: gas chromatography–mass spectrometry, OVA: Origanum vulgare of the Armenian highlands. |
3. Blossoming
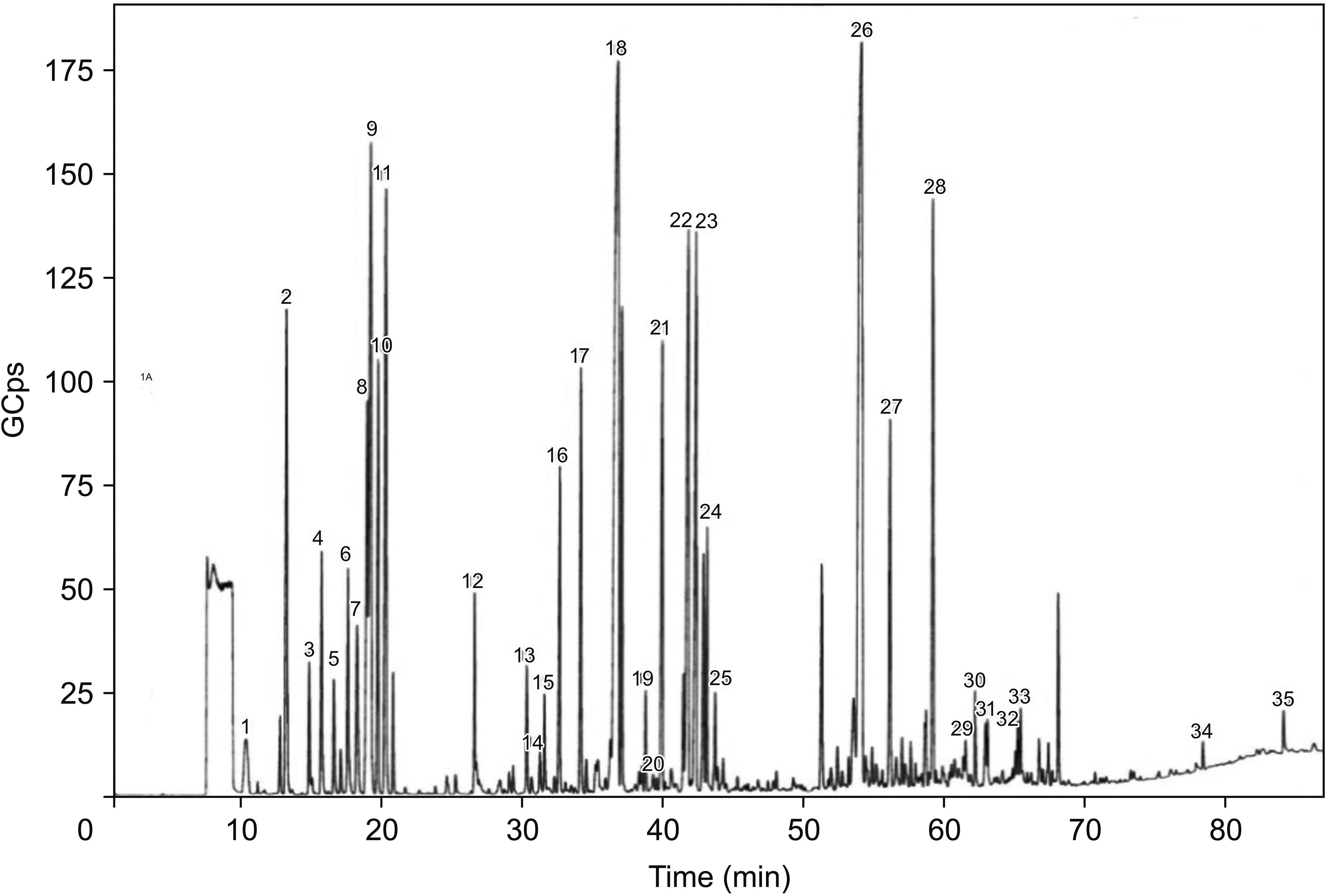 | Fig. 2GC-MS chromotograph of OVA essential oil in the period of blossoming. 1: β-Thujene, 2: (+)-Sabinene, 3: β-Pinene, 4: α-Terpinolene, 5: D-Limonene, 6: Eucalyptol, 7: γ-Terpinene, 8: Ethyl amyl ketone, 9: ο-Cymene, 10: 4-methyl-3-(1-methylethylidene)-1-cyclohexene, 11: 2-Hexenal diethyl acetal, trans, 12: 3-Octanol, 13: cis-β-Terpineol, 14: alfa-Copaene, 15: Dihydroedulan II, 16: (-)-β-Bourbonene, 17: β-Linalool, 18: β-Caryophyllene, 19: L-4-terpineol, 20: Alloaromadendrene, 21: Humulen, 22: Germacrene D, 23: I-β-Bisabolene, 24: Elixene, 25: α-trans-Farnesene, 26: β-Caryophyllene epoxide, 27: α-Humulene epoxide II, 28: Ent-Spathulenol, 29: Isoaromadendrene epoxide, 30: Carvacrol/Isothymol, 31: α-Cadinol, 32: Ledene oxide-(II), 33: Tetracyclo[6.3.2.0(2,5).0(1,8)]tridecan-9-ol,4,4-dimethyl-, 34: Dibutyl phthalate, 35: Palmitic acid. GC-MS: gas chromatography–mass spectrometry, OVA: Origanum vulgare of the Armenian highlands. |
4. Fruiting
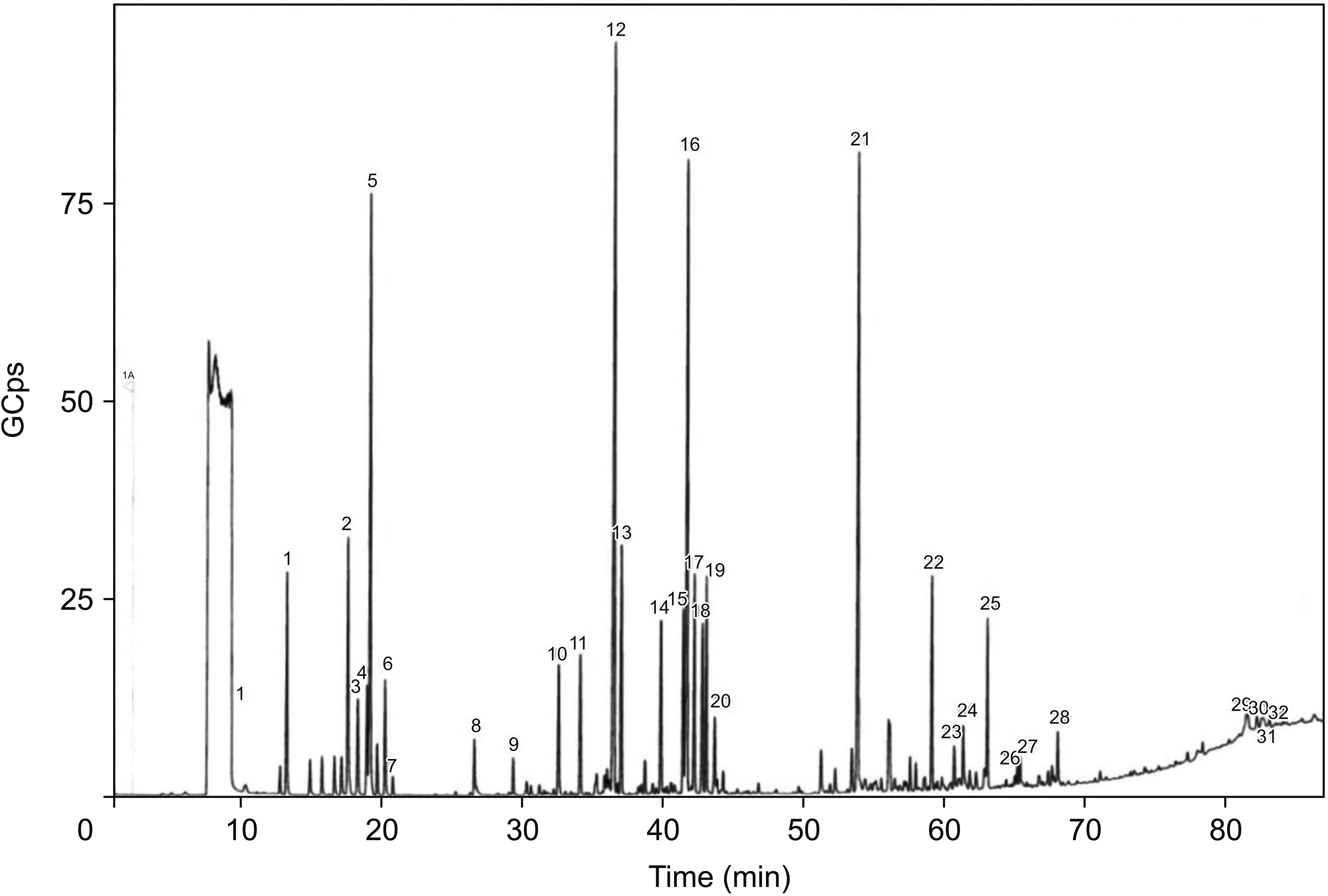 | Fig. 3GC-MS chromotograph of OVA essential oil in the period of fruiting. 1: Sabinene, 2: Eucalyptol, 3: trans-β-Ocimene, 4: γ-Terpinene, 5: β-Ocimene, 6: o-Cymene, 7: α-Terpinolene, 8: Ethylamylcarbinol, 9: Matsutake, 10: (-)-β-Bourbonene, 11: β-Linalool, 12: β-Caryophyllene, 13: L-4-terpineol, 14: α-Humulene, 15: α-Terpineol, 16: Germacrene D, 17: I-β-Bisabolene, 18: Elixene, 19: trans-α-Farnesene, 20: (+)-δ-Cadinene, 21: β-Caryophyllene epoxide, 22: Ent-Spathulenol, 23: tau-Cadinol, 24: 6-Isopropenyl-4,8a-dimethyl-3,5,6,7,8,8a-hexahydro-2-(1H)-naphthalenone, 25: α-Cadinol, 26: Tricyclo[5.2.2.0(1,6)]undecan-3-ol, 2-methylene-6,8,8-trimethyl, 27: Tetracyclo[6.3.2.0(2,5).0(1,8)]tridecan-9-ol,4,4-dimethyl, 28: trans-Z-α-Bisabolene epoxide, 29: 15,15`-Bi-1,4,7,10,13-pentaoxacyclohexadecane, 30: 3,6,9,12,15,18,21-Heptaoxatritriacontan-1-ol, 31: 3-Ethyl-5-(2-ethylbutyl)octadecane, 32: Isodecyl octyl phthalate. GC-MS: gas chromatography–mass spectrometry, OVA: Origanum vulgare of the Armenian highlands. |
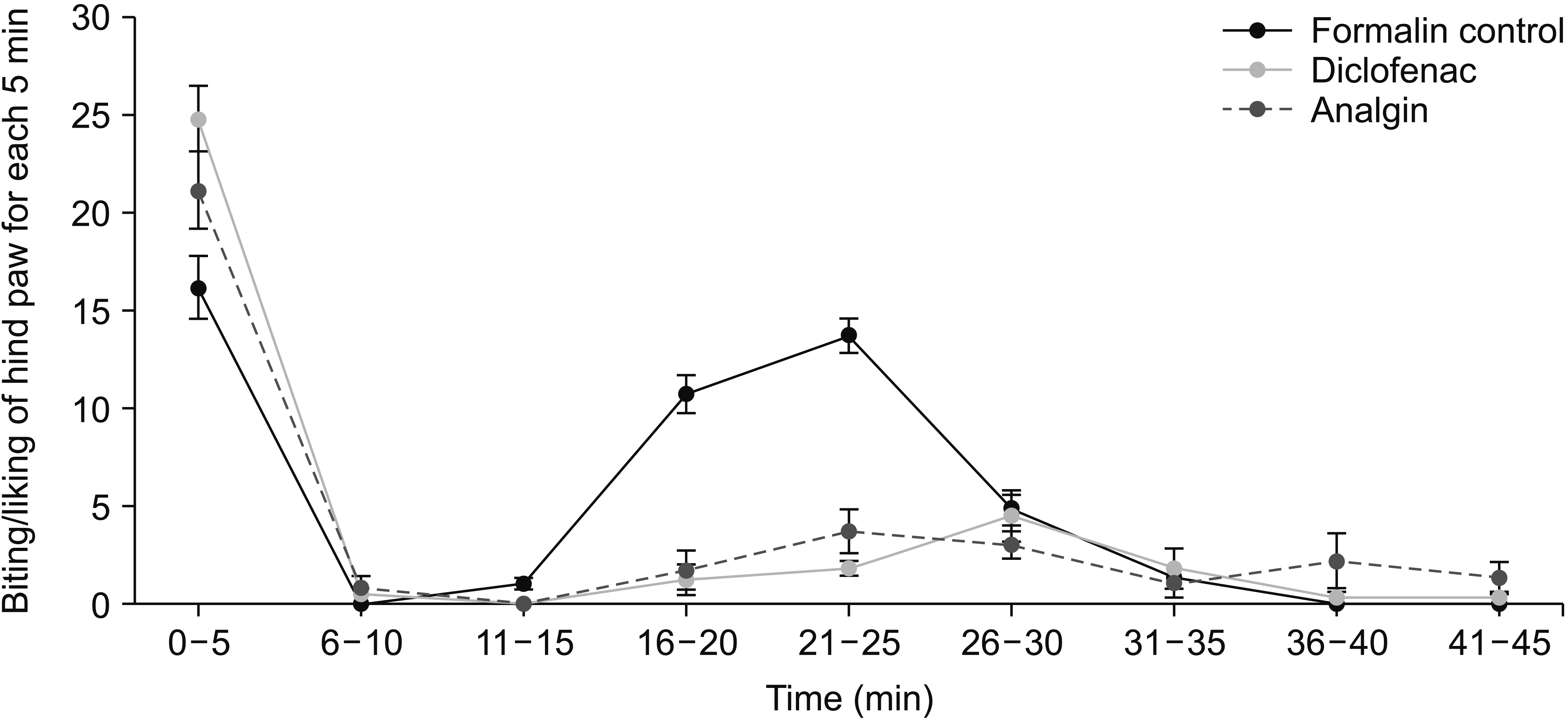
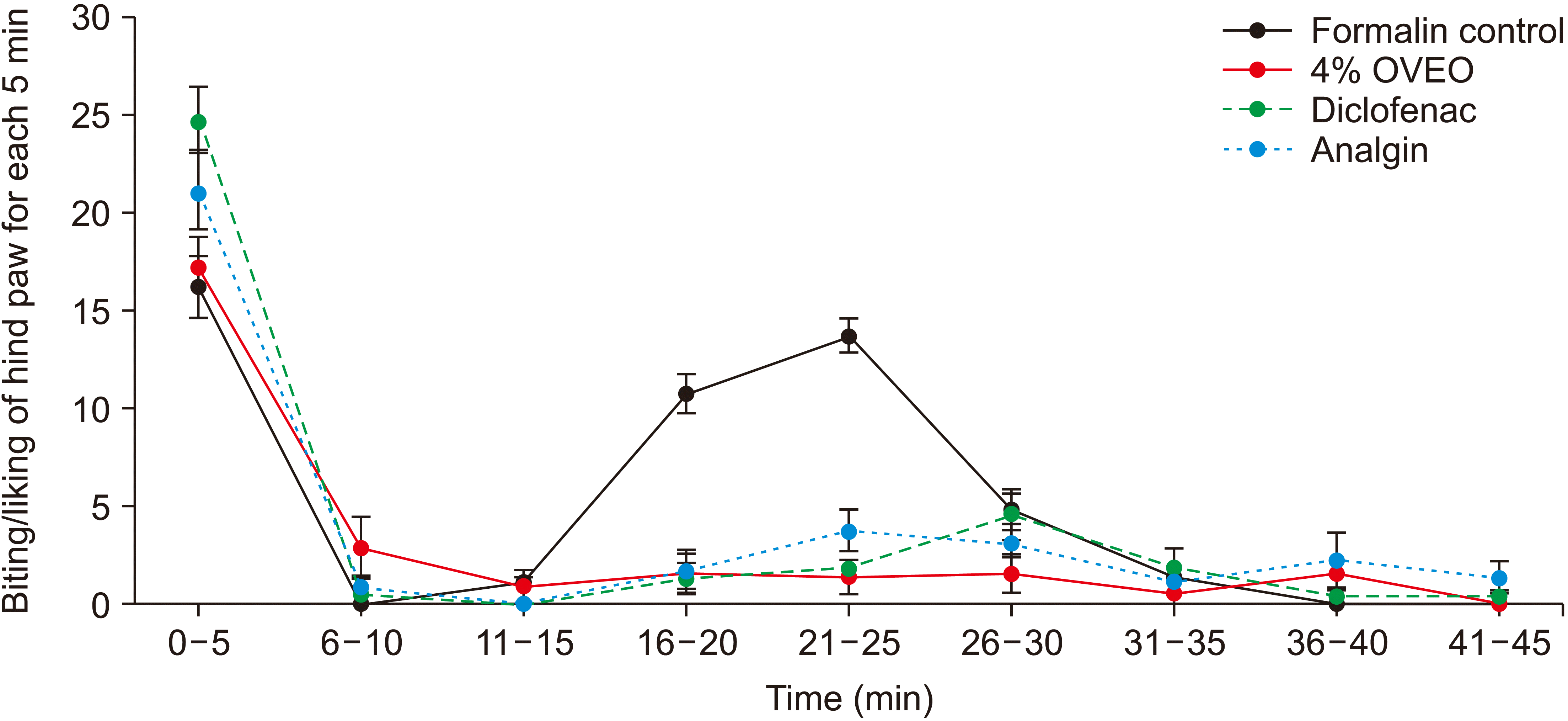
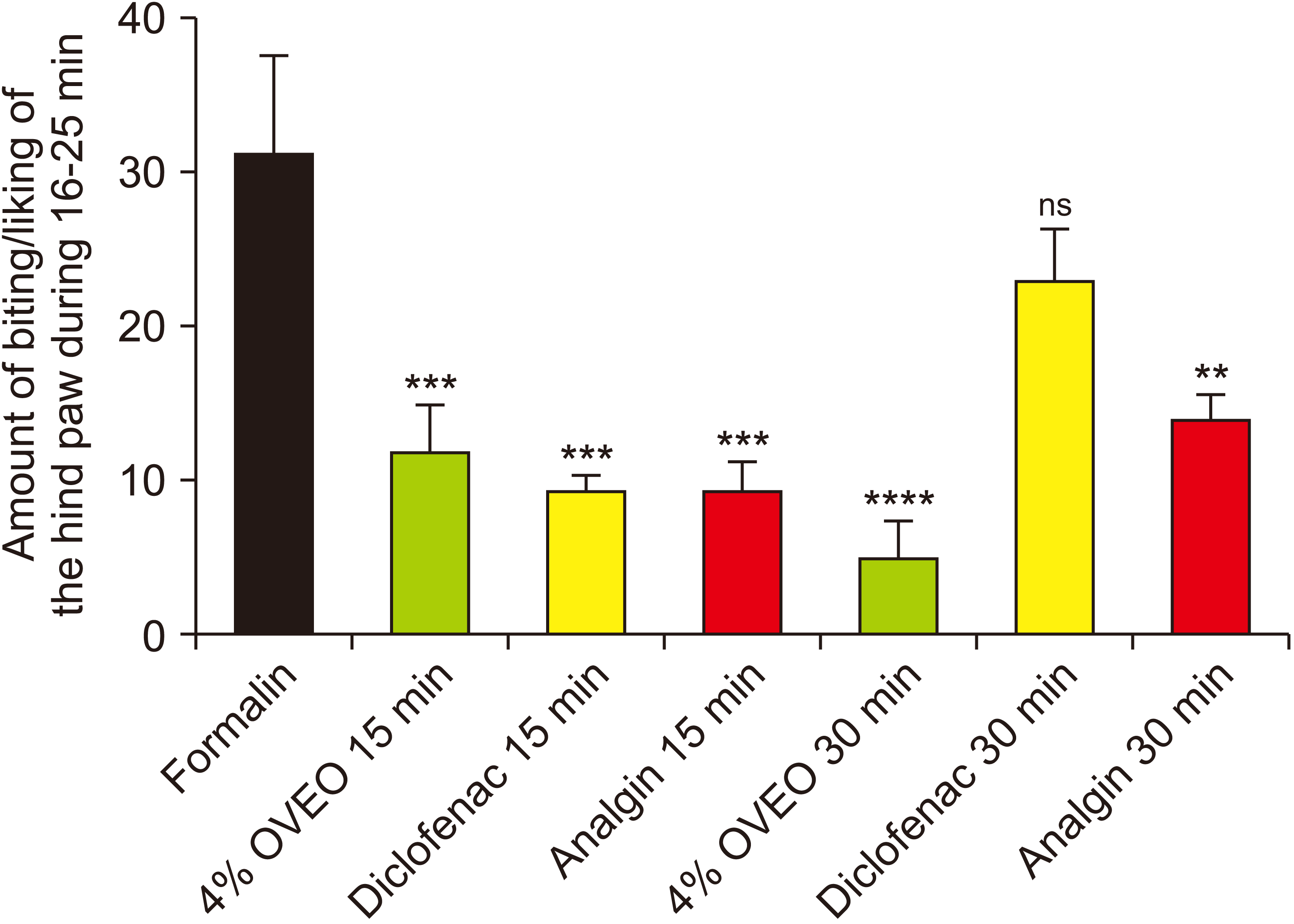
5. Analgesic and anti-inflammatory properties of OVA essential oil: the formalin test
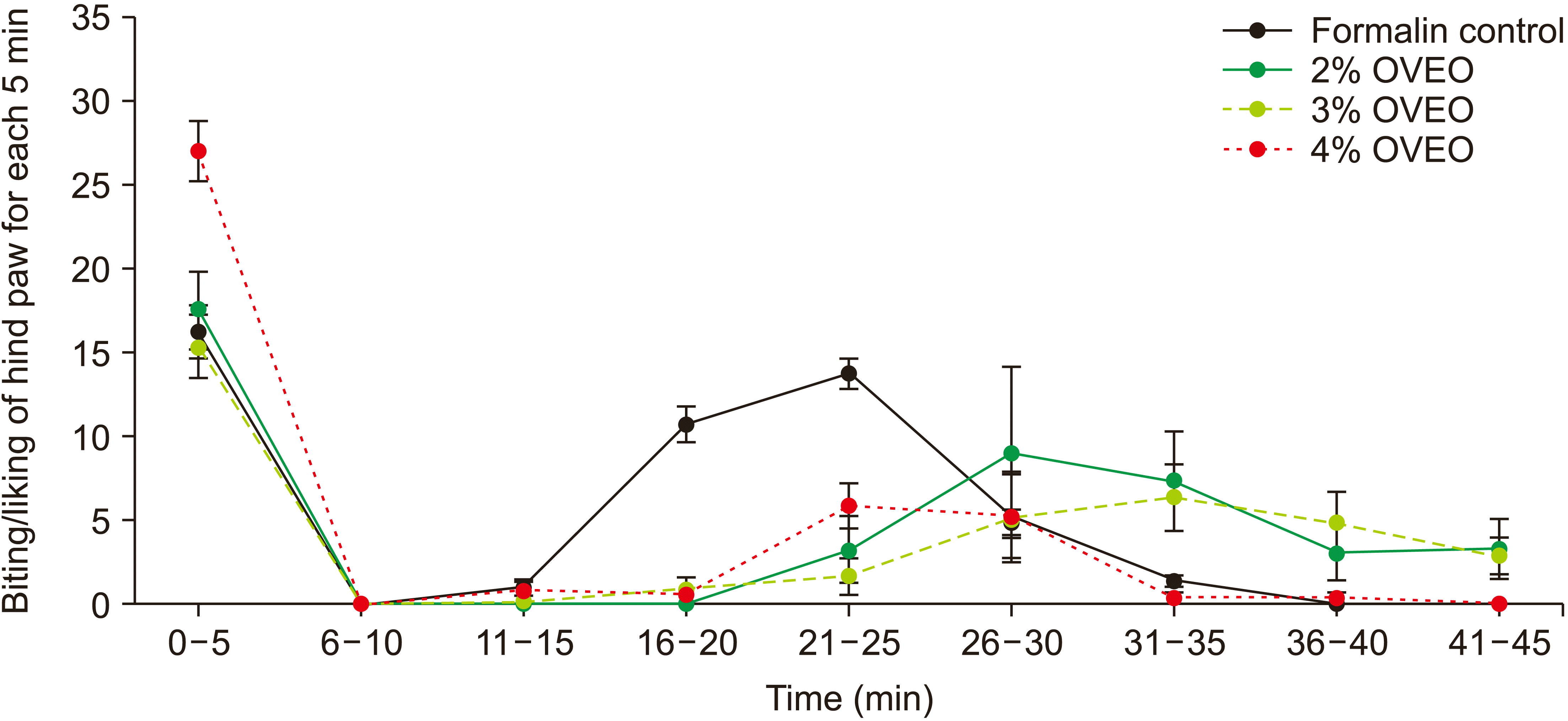 | Fig. 6The analgesic properties of various doses of OVA essential oil (OVEO), 15 minutes before formalin injection. The data represent mean ± standard error of mean. OVA: Origanum vulgare of the Armenian highlands. |
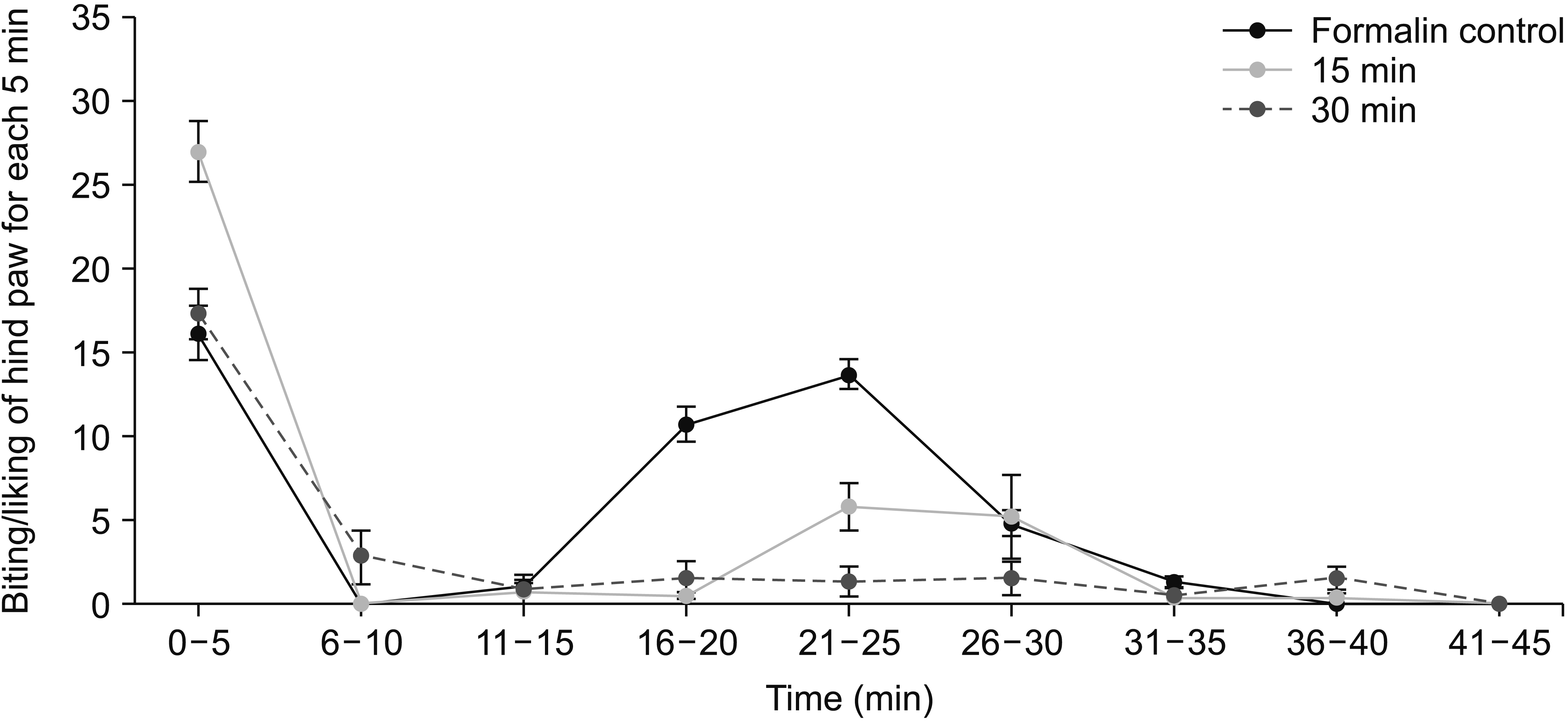 | Fig. 7The analgesic effect of 4% OVA essential oil 15 and 30 minutes before formalin injection. The data represent mean ± standard error of mean. OVA: Origanum vulgare of the Armenian highlands. |
6. Analgesic and anti-inflammatory properties of OVA essential oil: hot plate test
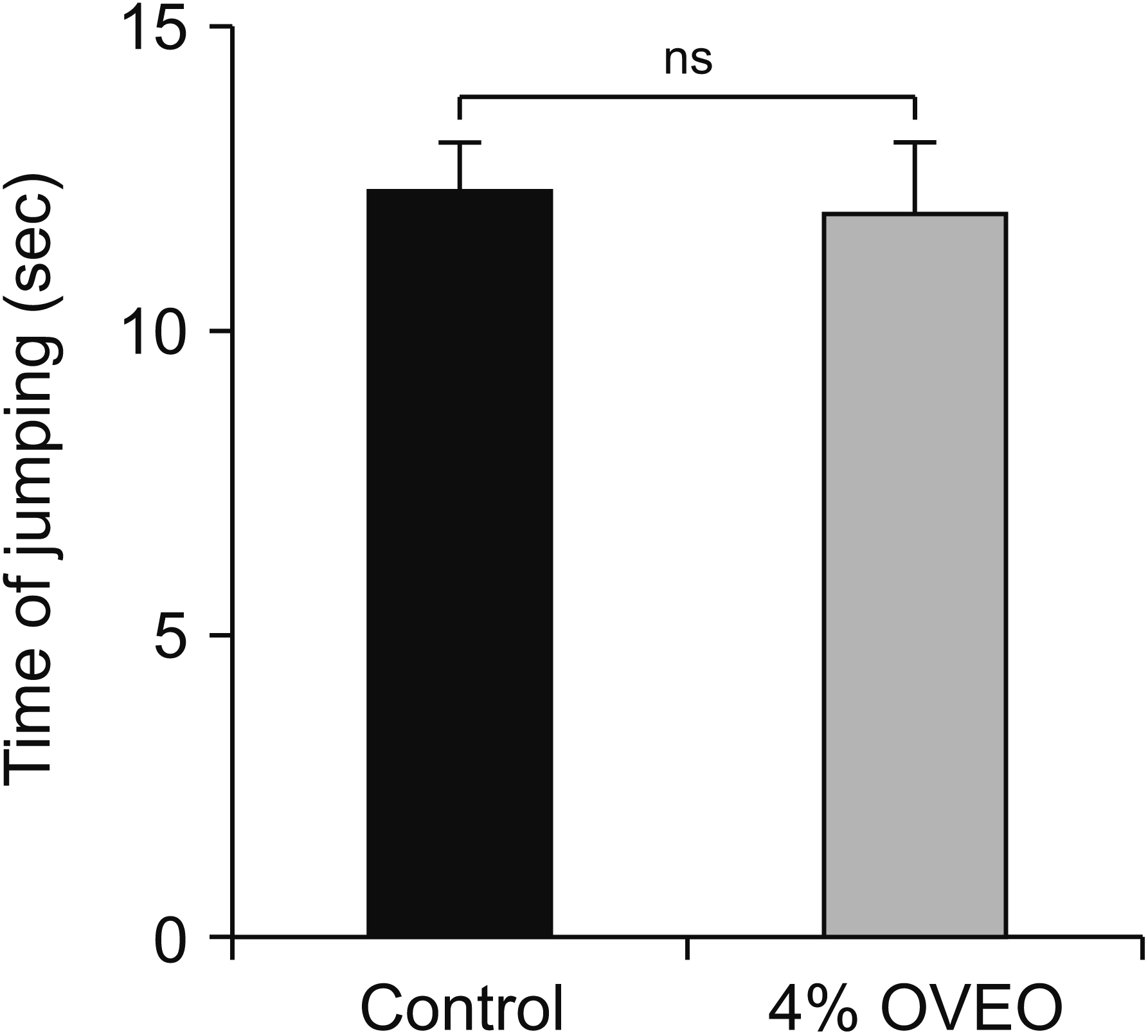 | Fig. 10Effect of the OVA essential oil in hot plate test in mice. Control (saline, 0.1 mL, intra peritoneal [IP]) and experimental (0.1 mL, 4% OVEO, IP) groups were injected 15 minutes before the behavioral test. The data represent mean ± standard error of mean. OVA: Origanum vulgare of the Armenian highlands, OVEO: OVA essential oil, ns: not significant. |
7. The cytotoxic properties of OVA essential oil: MTT assay
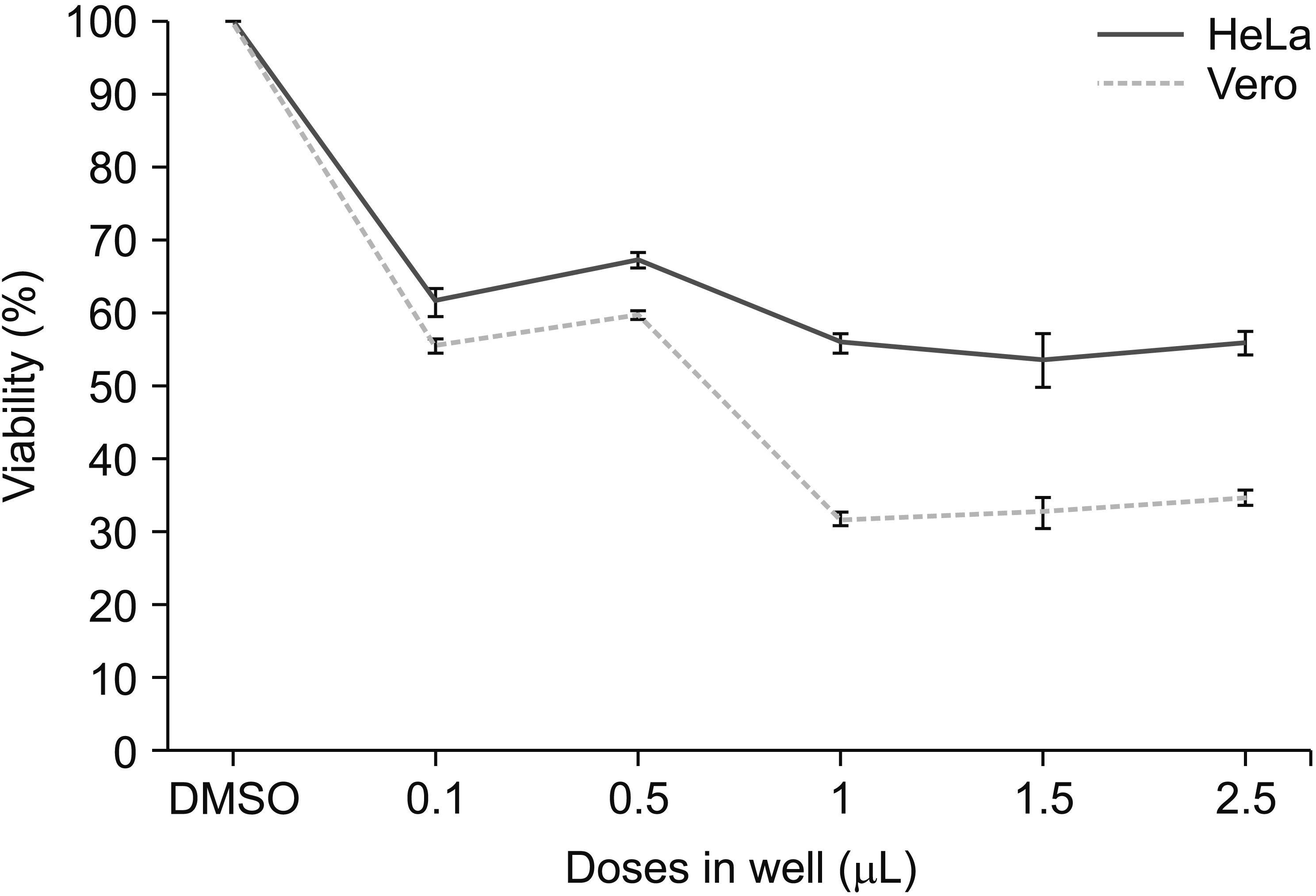 | Fig. 11The growth-inhibitory effect of OVA essential oil on HeLa and Vero cells viability (media with OVA essential oil preliminary addition). OVA: Origanum vulgare of the Armenian highlands. The data represent mean ± standard error of mean. |
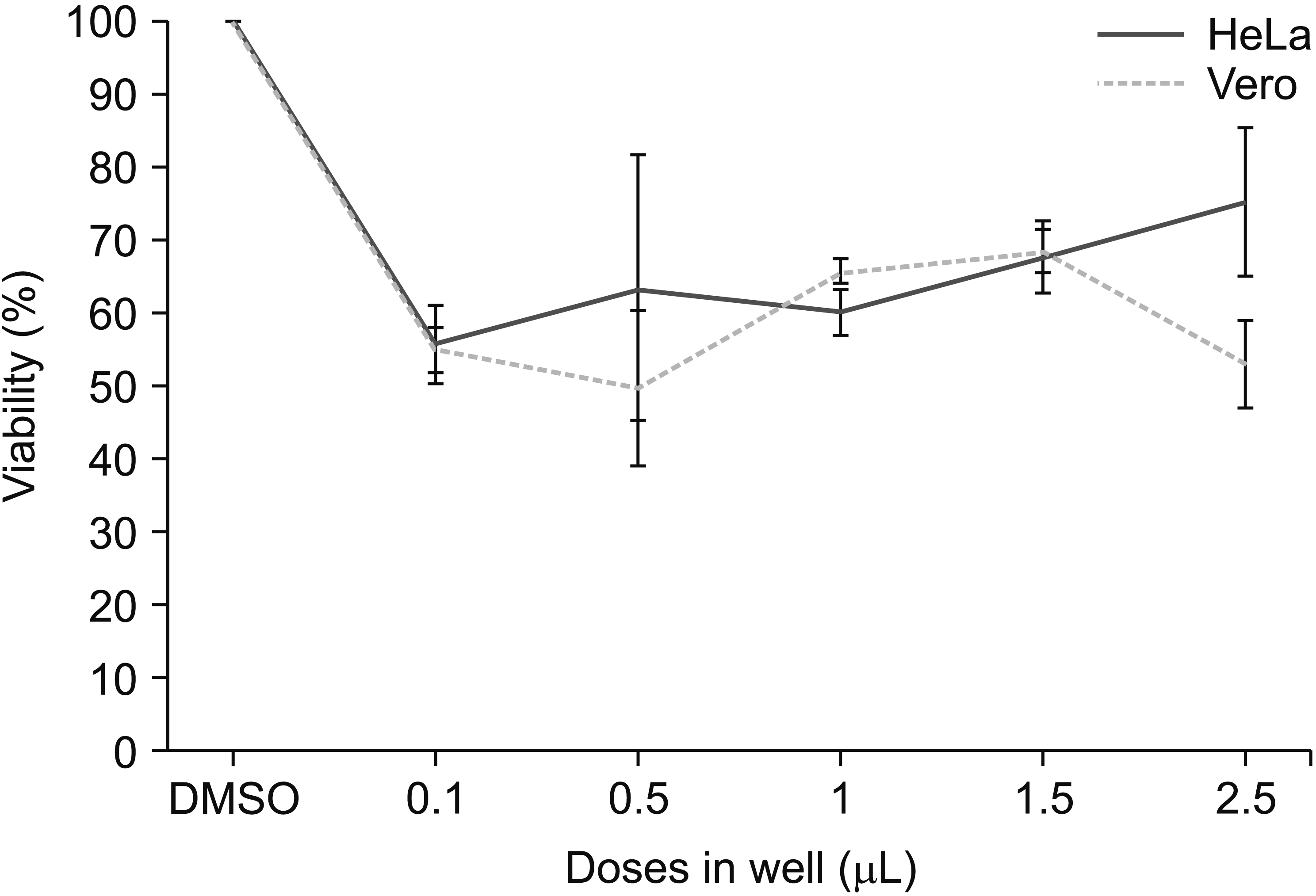 | Fig. 12The cytotoxic effect of OVA essential oil on HeLa and Vero cells viability (media without OVA essential oil addition). OVA: Origanum vulgare of the Armenian highlands. The data represent mean ± standard error of mean. |
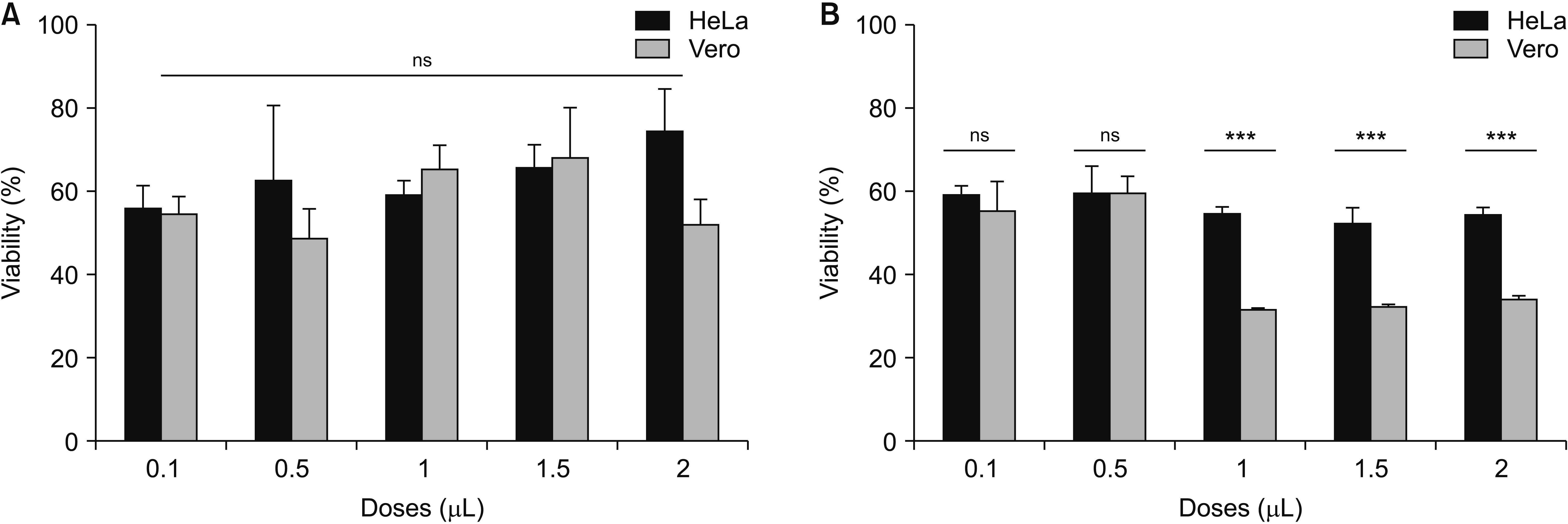 | Fig. 13Significance of cytotoxic effect of OVA essential oil. (A) The essential oil added after cell attachment. (B) The essential oil added before cell attachment (P < 0.05). The data represent mean ± standard error of mean. OVA: Origanum vulgare of the Armenian highlands, ns: not significant. ***P < 0.0005. |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download