Abstract
Progressive familial intrahepatic cholestasis (PFIC) is an autosomal recessive inherited disease requiring liver transplantation (LT). Hepatocellular carcinoma (HCC) is very rare in infants. We present a case of living donor LT using a left lateral section graft performed in a 7-month-old female infant diagnosed with PFIC type II and HCC. No mutation on ABCB11 gene was identified. Because of progressive deterioration of liver function, living donor LT with her mother’s left lateral section graft was performed. Pretransplant serum alpha-fetoprotein (AFP) level was increased to 2,740 ng/mL, but HCC was not taken into account because of its rarity. The explant liver showed micronodular liver cirrhosis, multiple infantile hemangiomas and two HCCs of 0.7 cm and 0.3 cm in size. The patient recovered uneventfully from the LT operation. This patient has been regularly followed up with abdomen ultrasonography and AFP measurement every 6 months. The patient has been continually doing well for 8 years after the LT. In conclusion, LT is currently the only effective treatment for PFIC-associated end-stage liver diseases. HCC can develop at the cirrhotic liver of any cause, thus elevation of HCC tumor markers in pediatric patients is an important clue to perform further investigation before LT.
Progressive familial intrahepatic cholestasis (PFIC) is an autosomal recessive inherited disease that disrupts genes encoding protein transporters responsible for bile formation [1]. Clinical manifestations in PFIC patients include progressive jaundice, pruritus, and growth retardation. Subsequently, patients will develop liver cirrhosis and hepatic failure. Liver transplantation (LT) has been regarded as the only effective treatment for PFIC.
In infant patients, hepatocellular carcinoma (HCC) is very rare. Most HCCs arise in the setting of prior liver disease. HCCs may be discovered incidentally in the explant or detected during surveillance of underlying disease, and it will trigger the decision to perform LT [2].
We herein present a case of living donor liver transplantation (LDLT) in a 7-month-old infant with PFIC and HCC using a left lateral section graft. The patient has been doing well for 8 years uneventfully.
This study was approved by the Institutional Review Board of Asan Medical Center (IRB No. 2020-0836), which waived the requirement for informed consent due to the retrospective nature of this study.
A 7-month-old female infant was referred to our institution for progressive jaundice. The patient was born through a full-term cesarean section delivery. Jaundice occurred after birth. It was not responsive to medical treatments. The patient was clinically diagnosed as PFIC type II, and no mutation on ABCB11 gene was identified. There were multiple enhancing nodules in both lobes of the liver, which were clinically diagnosed of infantile hemangioendothelioma (Fig. 1). Her jaundice progressed to have serum total bilirubin over 24 mg/dL. Coagulation profiles progressively deteriorated with frequent requirement of fresh frozen plasma transfusion. Interestingly, serum alpha-fetoprotein (AFP) level was increased to 2,740 ng/mL. The body weight of the patient was only 7 kg, thus the possibility of split LT was low within a short waiting period. Thus, we decided to perform LDLT under the clinical diagnosis of PFIC and hemangioendothelioma.
The donor was her 35-year-old mother, and the left lateral section graft weighed 216 g, being equivalent to a graft-to-recipient weight ratio of 3.1%. The graft left hepatic vein orifice was enlarged with patch venoplasty according to our institutional guidelines. Recipient operation was performed according to standard procedures of pediatric LDLT using a left lateral section graft. The native liver was cirrhotic, but there was no abnormality in the hilar vascular structures. Hepatic vein stumps of the recipient were unified to enlarge the orifice. Size-matched reconstruction of the graft hepatic vein was performed. Branch patch of the recipient portal vein was used for graft portal vein reconstruction. Single left hepatic artery reconstruction using surgical microscopy and Roux-en-Y hepaticojejunostomy were performed.
The explant liver showed micronodular liver cirrhosis, with cholestasis and bile plugs, giant cell transformation of hepatocytes, marked ductular proliferation and extramedullary hematopoiesis (Fig. 2). Overt liver cirrhosis with diffuse lobular fibrosis was identified. Immunohistochemical staining studies showed total loss of canalicular expression for bile salt export pump (BSEP) (Fig. 3). There were multiple infantile hemangiomas in both lobes of the liver with size up to 1.1 cm. In addition, there were two HCCs of 0.7 cm and 0.3 cm (Fig. 2). Tumor differentiation was Edmondson-Steiner grade II/II. Microvascular invasion, tumor necrosis, and satellite nodule were not identified.
Early follow-up computed tomography scan showed no vascular complications (Fig. 4). The patient recovered uneventfully from the LDLT operation. She has been regularly followed up with abdomen ultrasonography and AFP measurement every 6 months (Fig. 5). The patient has been doing well continually for 8 years after the transplantation.
LT has been accepted as the only effective treatment for PFIC patients with end-stage liver disease. Indications for LT include severe pruritus, significant growth retardation, liver cirrhosis, and liver failure. PFIC patients usually have growth retardation and metabolic bone disease, mainly related to impaired vitamin D and calcium absorption due to reduced bile secretion. Growth retardation can be significantly improved after LT [3,4]. In the present case, the patient also has a growth curve equivalent to normal percentiles.
PFIC is classified into three types (PFIC I-III): PFIC type I is caused by a mutation of the ATP8B1 gene encoding the FIC1 protein [1], PFIC type II is caused by a mutation of ABCB11 gene encoding the BSEP. PFIC type III is due to a mutation of ABCB4 gene encoding the MDR3 protein.
Hori et al. [9,10] have reported 14 patients who underwent LDLT for PFIC (11 cases of PFIC type I and 3 cases of PFIC type II). Three of the 11 PFIC type I recipients died, while all three PFIC type II recipients survived. Eight of the 11 PFIC type I recipients developed steatosis after LDLT. Nine of these 11 PFIC type I recipients developed fibrosis after LDLT. In contrast to PFIC type I recipients, PFIC type II recipients did not develop steatosis or fibrosis after LDLT. Liu et al have reported that two cases of PFIC type I developed steatohepatitis within 1 year after LT, thus suggesting the necessity of long-term follow-up [4]. PFIC type II patients with some subtypes are prone to recurrence of liver diseases [11].
In pediatric patients, HCC is a very rare disease. Most HCCs arise in the setting of prior liver diseases [2]. They might be diagnosed incidentally in the explant liver or detected during surveillance of underlying disease. Our present case showed a high level of AFP, but the diagnosis of HCC was not taken into account, and the multiple hepatic tumors were considered as infantile hemangioendothelioma. Infantile hepatic hemangioendothelioma is the most common hepatic vascular tumor in children [12]. In a single-center experience of 28 cases, the median age at diagnosis was 3 months. Eleven patients did not receive any treatment, whereas one patient underwent resectional surgery. Sixteen patients received drug treatment, nine of whom responded well to first-line propranolol/prednisolone, whereas seven patients needed salvage treatment. Twenty-five patients are alive, whereas three patients have died [12]. In 15 adult cases of hepatic epithelioid hemangioendothelioma who underwent hepatic resection or LT at our institution, no patient showed elevated AFP levels at all [13,14].
HCC has been rarely diagnosed in infant patients with biliary atresia or other disease-associated liver cirrhosis [5-8]. We identified multiple hepatic tumors in the present case, but HCC was not considered despite high rise of serum AFP because of the extreme rarity of HCC in infants. To the best of our knowledge, there are only five cases of HCC in infant LT recipients less than 1 year of age in the literature (Table 1).
In conclusion, LT is currently the only effective treatment for PFIC-associated end-stage liver diseases. HCC can develop at the cirrhotic liver of any cause, thus elevation of serum AFP in pediatric patients is an important clue to perform further investigation before LT.
ACKNOWLEDGMENTS
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Funding/Support
This study was supported by research grant from the Korean Society for Transplantation (2022-00-03002-004).
Author Contributions
Conceptualization: SH. Data curation: DYK, KMK, SHO. Methodology: JMN, CSA, HK, SH. Visualization: SH. Writing–original draft: SH, JMN. Writing–review & editing: SH.
REFERENCES
1. van Mil SW, Klomp LW, Bull LN, Houwen RH. 2001; FIC1 disease: a spectrum of intrahepatic cholestatic disorders. Semin Liver Dis. 21:535–44. DOI: 10.1055/s-2001-19034. PMID: 11745041.

2. Sindhi R, Rohan V, Bukowinski A, Tadros S, de Ville de Goyet J, Rapkin L, et al. 2020; Liver transplantation for pediatric liver cancer. Cancers (Basel). 12:720. DOI: 10.3390/cancers12030720. PMID: 32204368. PMCID: PMC7140094.

3. Aydogdu S, Cakir M, Arikan C, Tumgor G, Yuksekkaya HA, Yilmaz F, et al. 2007; Liver transplantation for progressive familial intrahepatic cholestasis: clinical and histopathological findings, outcome and impact on growth. Pediatr Transplant. 11:634–40. DOI: 10.1111/j.1399-3046.2007.00722.x. PMID: 17663686.

4. Liu Y, Sun LY, Zhu ZJ, Wei L, Qu W, Zeng ZG. 2018; Liver transplantation for progressive familial intrahepatic cholestasis. Ann Transplant. 23:666–73. DOI: 10.12659/AOT.909941. PMID: 30250015. PMCID: PMC6248029.

5. Esquivel CO, Gutiérrez C, Cox KL, Garcia-Kennedy R, Berquist W, Concepcion W. 1994; Hepatocellular carcinoma and liver cell dysplasia in children with chronic liver disease. J Pediatr Surg. 29:1465–9. DOI: 10.1016/0022-3468(94)90145-7. PMID: 7844722.

6. Brunati A, Feruzi Z, Sokal E, Smets F, Fervaille C, Gosseye S, et al. 2007; Early occurrence of hepatocellular carcinoma in biliary atresia treated by liver transplantation. Pediatr Transplant. 11:117–9. DOI: 10.1111/j.1399-3046.2006.00623.x. PMID: 17239135.

7. Iida T, Zendejas IR, Kayler LK, Magliocca JF, Kim RD, Hemming AW, et al. 2009; Hepatocellular carcinoma in a 10-month-old biliary atresia child. Pediatr Transplant. 13:1048–9. DOI: 10.1111/j.1399-3046.2008.01094.x. PMID: 19032418.

8. Kim JM, Lee SK, Kwon CH, Joh JW, Choe YH, Park CK. 2012; Hepatocellular carcinoma in an infant with biliary atresia younger than 1 year. J Pediatr Surg. 47:819–21. DOI: 10.1016/j.jpedsurg.2012.01.020. PMID: 22498405.

9. Hori T, Egawa H, Miyagawa-Hayashino A, Yorifuji T, Yonekawa Y, Nguyen JH, et al. 2011; Living-donor liver transplantation for progressive familial intrahepatic cholestasis. World J Surg. 35:393–402. DOI: 10.1007/s00268-010-0869-6. PMID: 21125272.

10. Hori T, Egawa H, Takada Y, Ueda M, Oike F, Ogura Y, et al. 2011; Progressive familial intrahepatic cholestasis: a single-center experience of living-donor liver transplantation during two decades in Japan. Clin Transplant. 25:776–85. DOI: 10.1111/j.1399-0012.2010.01368.x. PMID: 21158920.

11. Maggiore G, Gonzales E, Sciveres M, Redon MJ, Grosse B, Stieger B, et al. 2010; Relapsing features of bile salt export pump deficiency after liver transplantation in two patients with progressive familial intrahepatic cholestasis type 2. J Hepatol. 53:981–6. DOI: 10.1016/j.jhep.2010.05.025. PMID: 20800306.

12. Emad A, Fadel S, El Wakeel M, Nagy N, Zamzam M, Kieran MW, et al. 2020; Outcome of children treated for infantile hepatic hemangioendothelioma. J Pediatr Hematol Oncol. 42:126–30. DOI: 10.1097/MPH.0000000000001536. PMID: 31233466.

13. Na BG, Hwang S, Ahn CS, Kim KH, Moon DB, Ha TY, et al. 2021; Prognosis of hepatic epithelioid hemangioendothelioma after living donor liver transplantation. Korean J Transplant. 35:15–23. DOI: 10.4285/kjt.20.0049.

14. Na BG, Hwang S, Ahn CS, Kim KH, Moon DB, Ha TY, et al. 2021; Post-resection prognosis of patients with hepatic epithelioid hemangioendothelioma. Ann Surg Treat Res. 100:137–43. DOI: 10.4174/astr.2021.100.3.137. PMID: 33748027. PMCID: PMC7943284.

Fig. 1
Pretransplant computed tomography scan taken at 1 month before liver transplantation. There are multiple enhancing nodules scattered at both lobes of the liver, which are enhanced in the arterial phase images (A, C) and portal phase images (B, D).
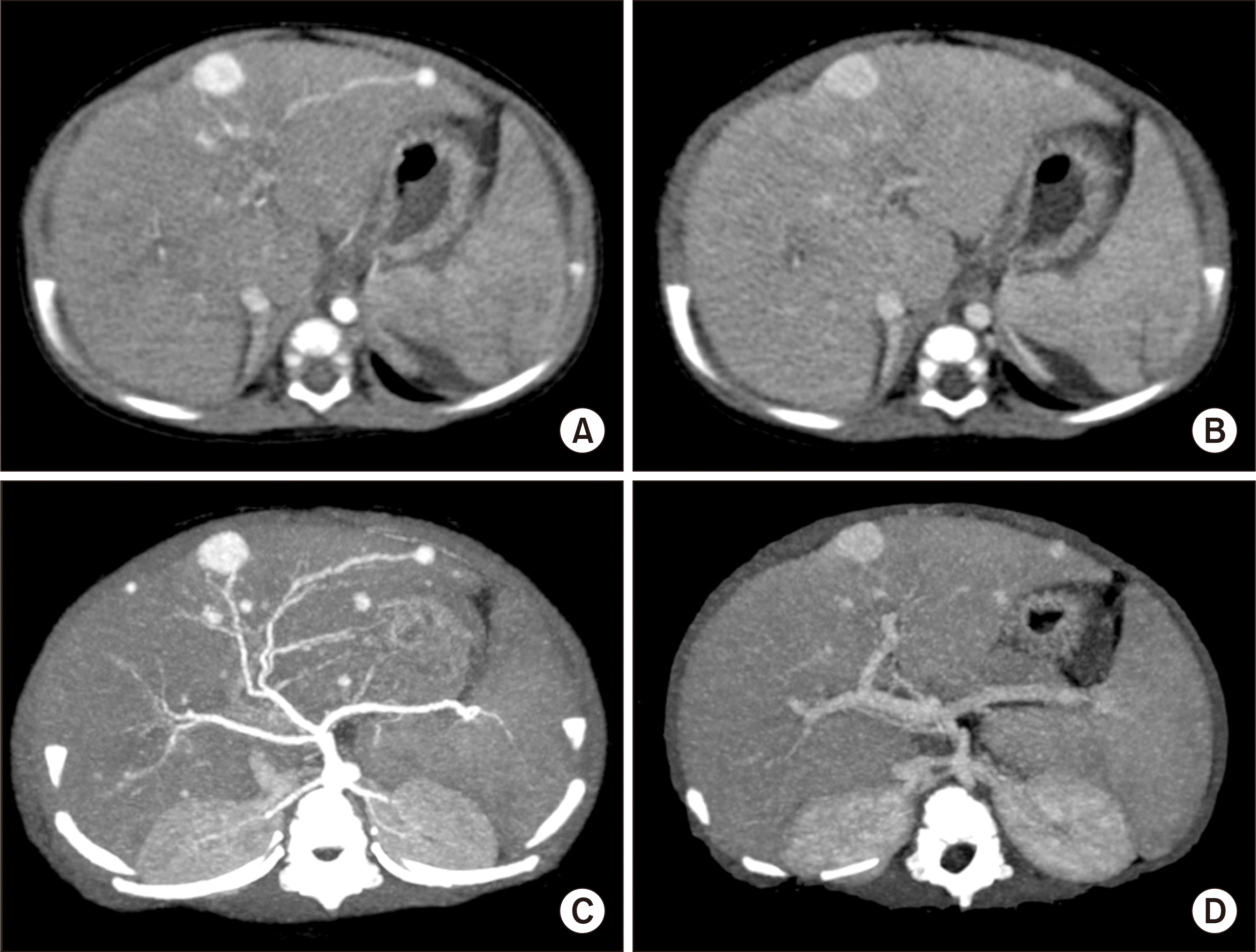
Fig. 2
Gross photograph of the explant liver showing micronodular liver cirrhosis (A) and pathology-proven hepatocellular carcinoma (B, arrow).
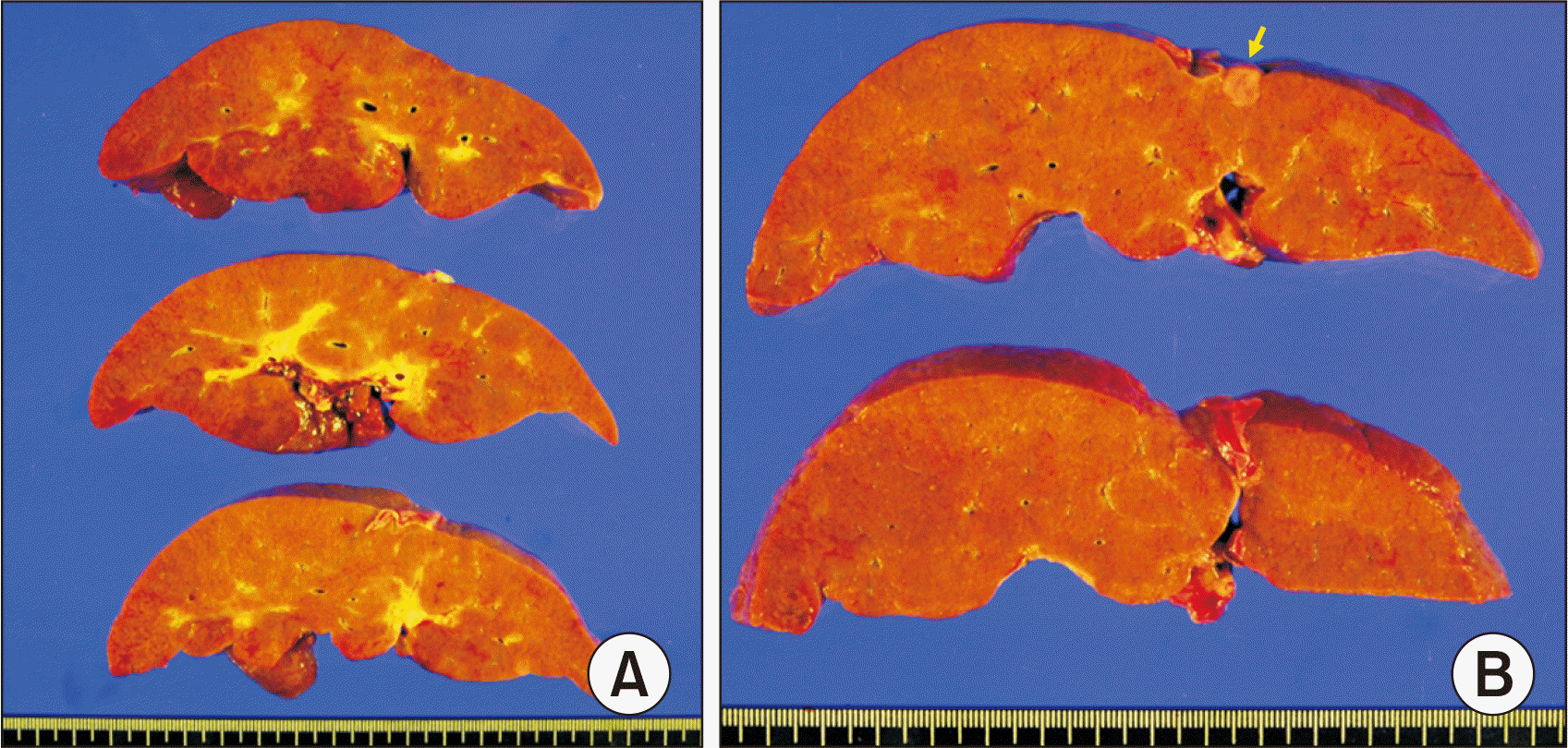
Fig. 3
Microscopic findings of the explant liver. (A) Histologic study shows bile duct proliferation with cholangiolar cholestasis (H&E, ×100). (B) Liver cirrhosis with diffuse lobular fibrosis is identified (Masson trichrome staining, ×50). (C) Immunohistochemical staining study for bile salt export pump (BSEP) showing total loss of canalicular expression (BSEP staining, ×200). (D) Normal control specimen showing normal canalicular expression for BSEP (BSEP staining, ×200).
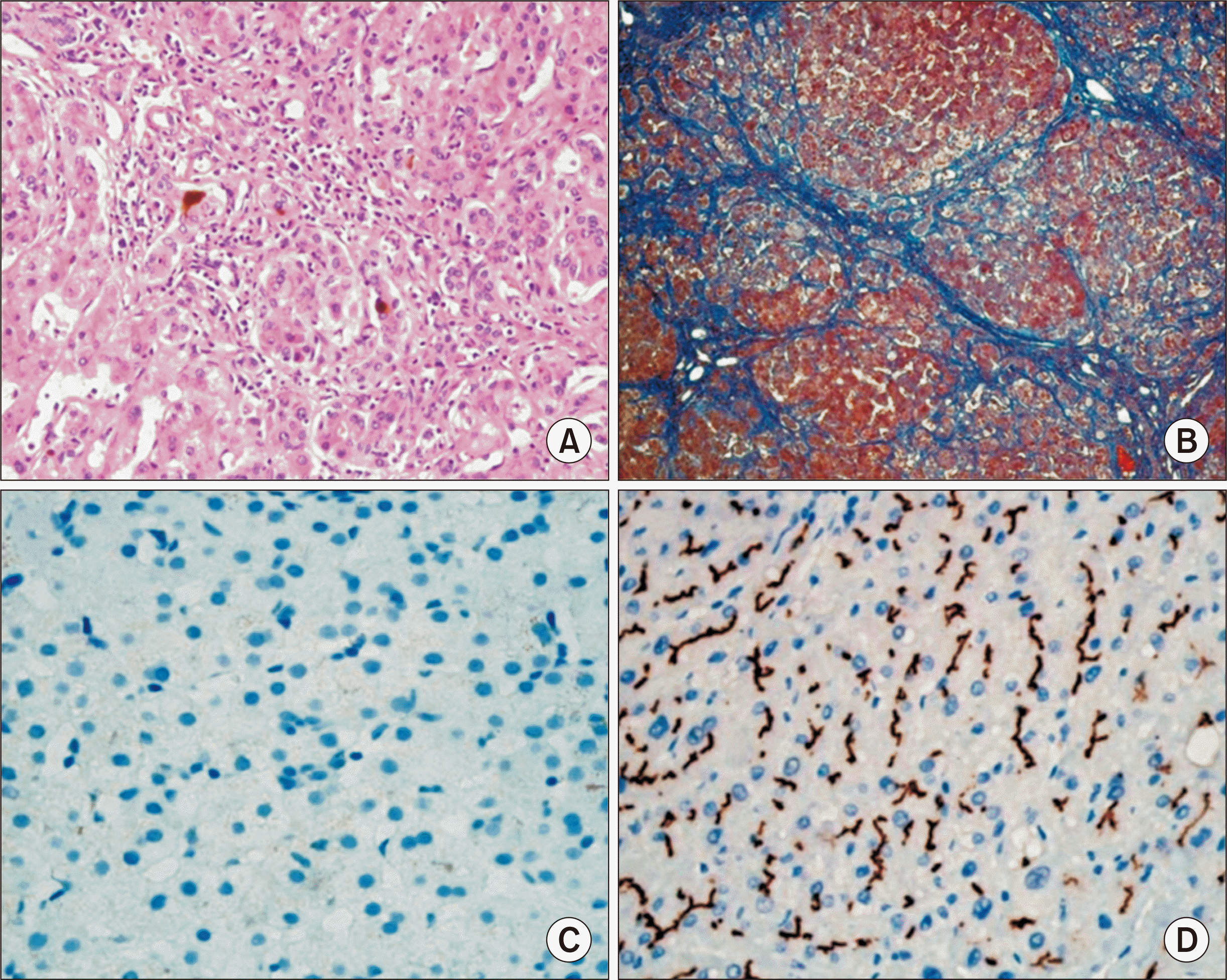
Fig. 4
Posttransplant computed tomography scans taken at 4 days (A) and 1 year (B) after liver transplantation. No abnormal finding is identified.
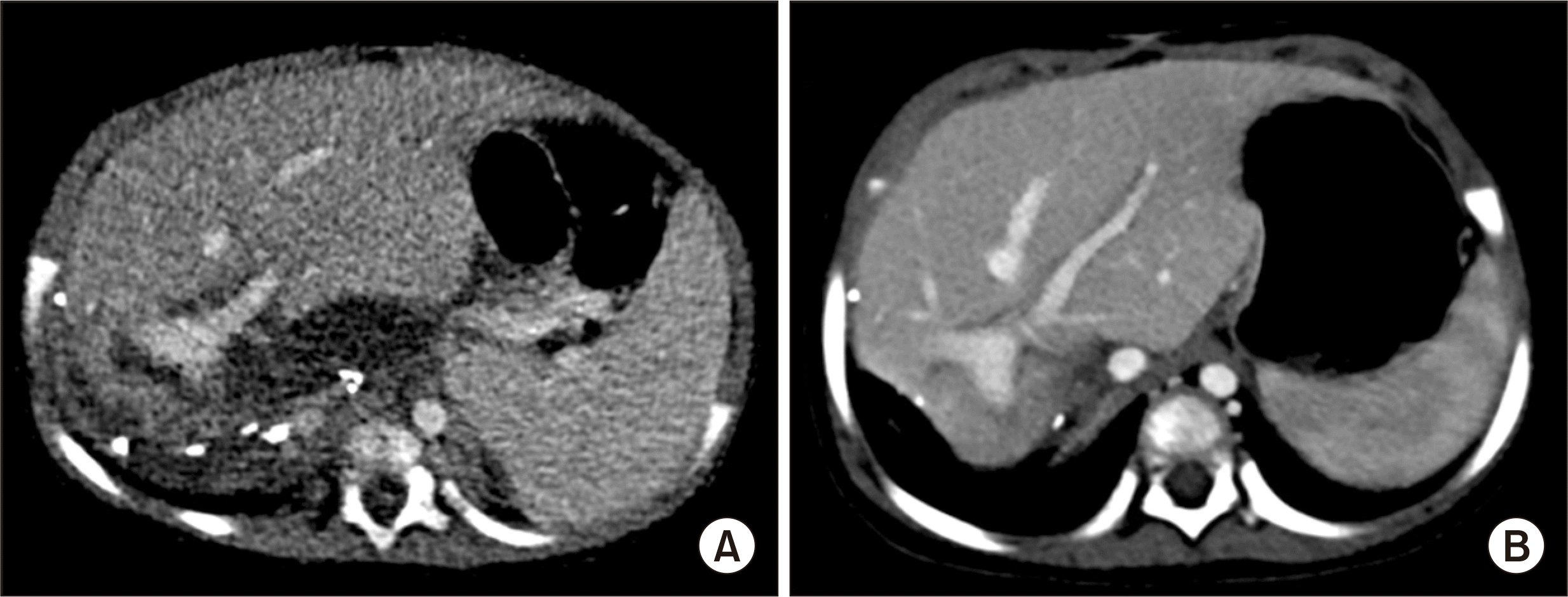
Fig. 5
Posttransplant Doppler ultrasonography follow-up images taken at 2 years (A), 4 years (B), 6 years (C), and 8 years (D) after transplantation. No abnormal finding is identified.
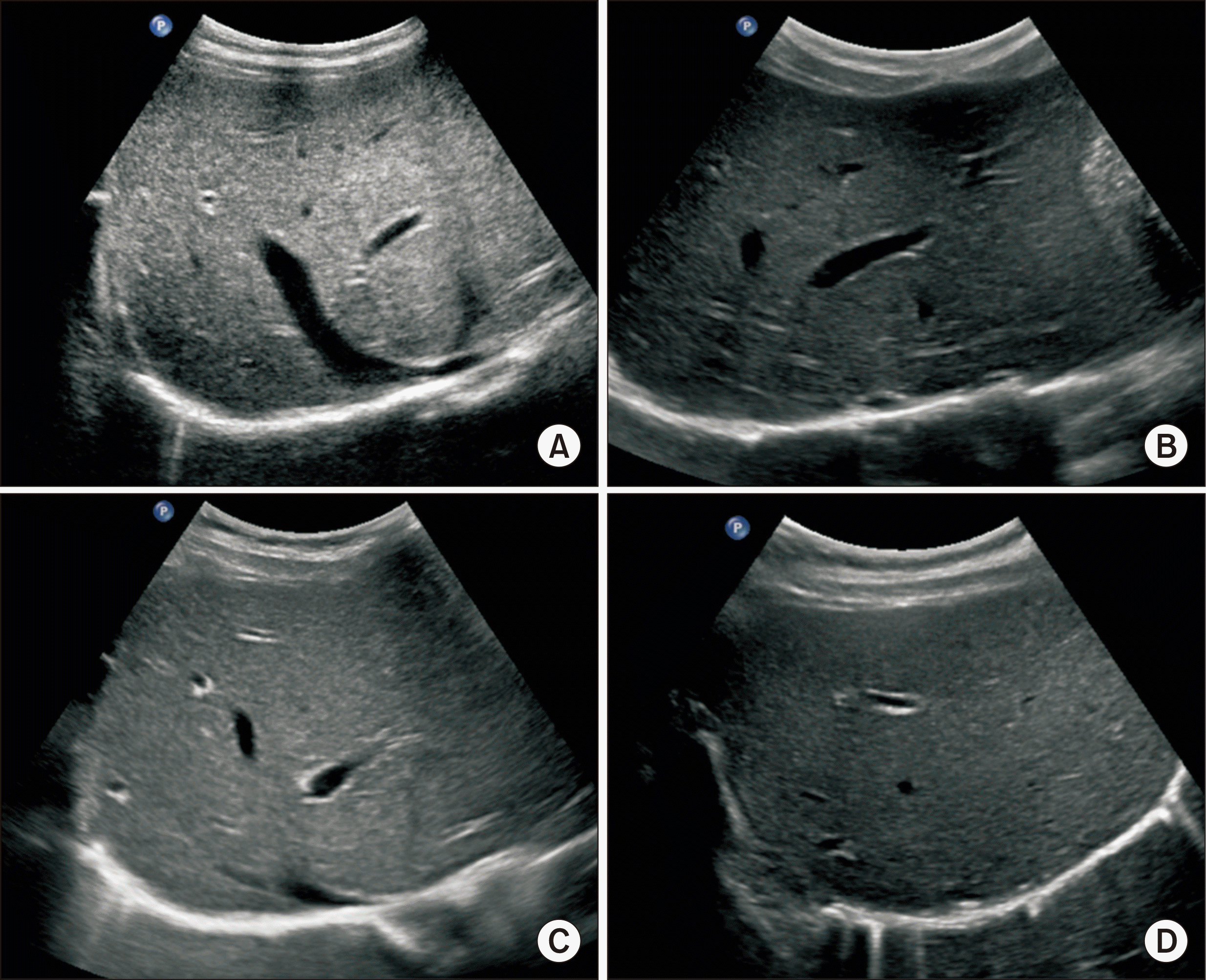
Table 1
Collective review of infant liver transplant recipients diagnosed with HCC
| Study | Age at HCC diagnosis/LT (mo) | Liver disease | Tumor number/size (cm) | LT type | Follow-up outcome |
|---|---|---|---|---|---|
| Esquivel et al. (1994) [5] | 0.2/2 | Neonatal iron storage disease | 1/0.2 | DDLT | 29 mo, dead, NED |
| Brunati et al. (2007) [6] | 2/8 | BA | 1/0.5 | DDLT | 12 mo, alive, NED |
| Iida et al. (2009) [7] | 2/10 | BA | 1/0.7 | DDLT | 4 mo, alive, NED |
| Kim et al. (2012) [8] | 10/10 | BA | 1/0.9 | LDLT | 4 mo, alive, NED |
| This study (2013) | 7/7 | PFIC | 2/0.7, 0.3 | LDLT | 96 mo, alive, NED |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download