A 59-year-old man was referred to our hospital due to condition of post-iñuenza pneumonia and pancytopenia. At the time of admission, he had a fever, but there was no recent reports of weight loss or night sweats. Complete blood cell count at the time of admission was: hemoglobin 9.1 g/dL (reference range 13.0–18.0 g/dL), white blood cells 1.07×10
9/L (reference range 4.0–10.0×10
9/L), absolute neutrophils 0.35×10
9/L (reference range 1.4–7.5×10
9/L), platelets 12×10
9/L (reference range 150–400×10
9/L), and absolute reticulocytes 9.1×10
9/L (reference range 24–84×10
9/L). His total protein level was 6.3 g/dL (reference range 5.8–8.1 g/dL), albumin level was 3.7 g/dL (reference range 3.1–5.2 g/dL), and albumin/globulin ratio was 1.4 (reference range 1.2–2.1), which were all normal. However, his C-reactive protein level was elevated (210.01 mg/L, reference range 0–5.0 mg/L). Proteinuria (542.34 mg/day) was detected in urine analysis. M protein was also detected in the serum (0.08 g/dL, Ig G lambda type,
Fig. 1), but not in the urine in capillary protein electrophoresis (EP) and capillary immunofixation electrophoresis (IFE) analyses. His serum and urine free light chain concentrations were as follows: free kappa in the serum 35.93 mg/dL (reference range 3.30–19.40 mg/dL) and urine 586.01 mg/dL (reference range 0.78–13.48 mg/dL), and free lambda in the serum 41.66 mg/dL (reference range 5.71–26.30 mg/dL) and in the urine 217.28 mg/dL (reference range 2.22–5.90 mg/dL). The kappa/lambda free light chain ratios were all within the normal limits (0.86 for serum and 2.70 for urine). There was no splenomegaly or enlarged lymph nodes observed in the chest and abdomen computed tomography scans. Bone marrow (BM) examination was performed to evaluate the cause of presence of M protein and pancytopenia, which revealed hypocellular marrow (5% of cellularity,
Fig. 2A) with an increase in plasma cell proportion (27.4%,
Fig. 2A and B). The immunohistochemical (IHC) staining for CD138, kappa, lambda, and CD56 did not reveal monoclonal or aberrant plasma cells in the BM biopsy (
Fig. 2B–D). The ˜ow cytometric (FCM) immunophenotyping with BM aspirate was performed using a myeloma panel consisting of CD19, CD28, CD38, CD45, CD56, CD117, CD138, kappa, and lambda light chain. CD38 and CD138 positive plasma cells were also found to be positive for CD19 and negative for CD56, CD28, and CD117. So, a single population of normal plasma cells without light chain restriction was confirmed (
Fig. 2E and F). The diagnosis of severe AA was confirmed and follow-up serum protein EP and IFE tests were recommended. The patient was subjected to the empirical pneumonia antibiotic therapy. However, he died due to the aggravation of pneumonia and multiorgan failure on day 19 of his hospital stay.
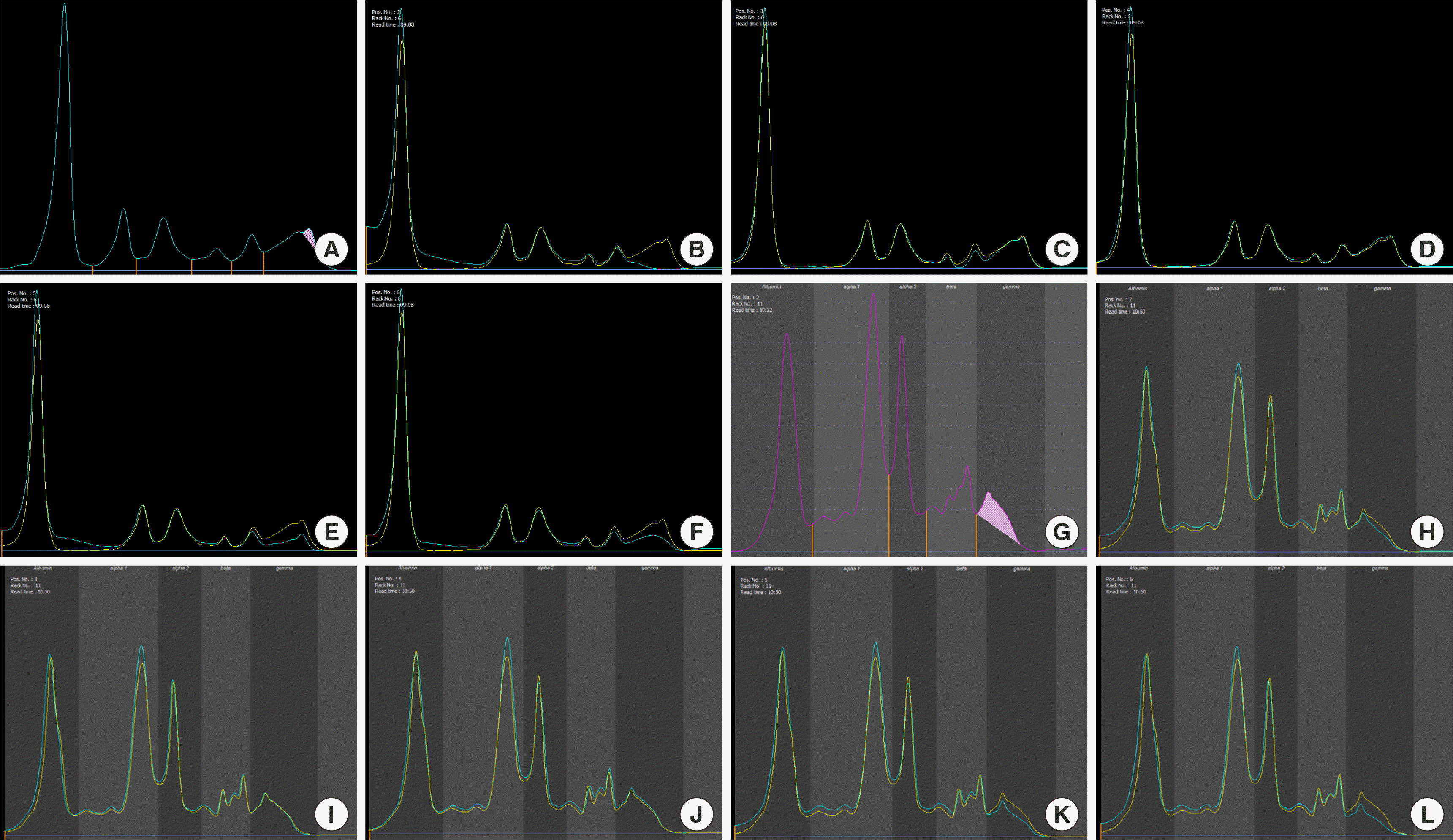 | Fig. 1Capillary electrophoresis (EP) and capillary immunofixation electrophoresis (IFE) of the serum and urine samples. (A) Serum EP. (B-F) Serum IFE: (B) IgG, (C) IgA, (D) IgM, (E) Kappa light chain, and (F) Lambda light chain. (G) Urine EP. (H-L) Urine IFE: (H) IgG, (I) IgA, (J) IgM, (K) Kappa light chain, and (L) Lambda light chain. 
|
 | Fig. 2Immunohistochemical (IHC) staining of the bone marrow (BM) biopsy specimen and multicolor flow cytometric (FCM) immunophenotyping of the plasma cells. (A) Hematoxylin and eosin staining (×200). (B-D) IHC staining of (B) CD 138, (C) kappa light chain, and (D) lambda light chain (×400). (E, F) FCM immunophenotyping of plasma cells in BM aspirate. (G) Result of the lymphocyte subset analysis of BM aspirate using FCM panel corresponding to plasma cell myeloma. 
|






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download