Abstract
Background
Thromboelastography (TEG) is a method that assesses whole-blood coagulation efficiency by measuring different parameters of clot formation and clot lysis. Whether and how blood coagulation factors influence the TEG parameters is unknown.
Methods
Blood coagulation factors measured by an automated coagulation analyzer (ACL TOP; Beckman Coulter, USA) and thrombin generation measured by automated thrombogram method (Thrombinoscope, Netherlands) were quantified in 109 healthy volunteers to investigate their correlation with six TEG parameters. TEG parameters were analyzed with the TEG 5000 Hemostasis Analyzer System (Haemonetics, USA). To assess whether TEG detects coagulation factor deficiencies, TEG was performed in four patients, with three known coagulation factor deficiencies.
Results
Reaction time negatively correlated with factors V and XII, and clot formation time positively correlated only with factor XII. Alpha angle positively correlated with factor XII and negatively with protein S. Maximum amplitude showed a positive correlation with fibrinogen and factors VIII and XII, whereas coagulation index only with factors VIII and XII. Thrombin generation and percentage of fibrinolysis at 30 minutes after maximum amplitude exhibited no correlation with the TEG parameters. Of the four TEG-tested patients, only the patient with factor V deficiency had elevated reaction time values.
초록
배경
점탄성응고검사(thromboelastography, TEG)는 혈전 형성과 혈전 용해의 다양한 매개변수를 측정하여 전혈의 응고효율을 평가하는 방법이다. 혈액 응고인자가 TEG 매개변수에 영향을 주는지 여부 및 영향을 미치는 방법은 알려져 있지 않다.
방법
109명의 건강한 지원자를 대상으로 혈액응고 인자를 혈액응고 자동분석기로 측정하고 트롬빈 생성을 자동 트롬보그램으로 정량하여 6개의 TEG 매개변수와 상관관계를 구하였다. TEG가 응고인자의 결핍을 탐지할 수 있는지 평가하기 위해 세 가지의 응고인자 결핍이 있는 네 명의 환자들에게서 TEG 검사를 시행하였다.
결과
반응 시간(R)은 응고인자 V 및 XII와 음의 상관관계가 있었고 혈전 형성 시간(K)은 응고인자 XII와만 양의 상관관계가 있었다. 알파 각도(α)는 응고인자 XII와는 양의 상관관계가 있었으며 단백질 S와는 음의 상관관계가 있었다. 최대 진폭(MA)은 피브리노겐 및 응고인자 VIII, XII와 양의 상관관계를 보인 반면 응고지수(CI)는 응고인자 VIII과 XII에만 양의 상관관계를 보였다. 트롬빈 생성 및 LY30은 TEG 매개변수와 상관관계를 갖지 않았다. TEG 검사를 받은 4명의 환자 중에서는, 응고인자 V 결핍증이 있는 환자에서만 반응시간(R)이 상승했다.
Viscoelastic tests, such as thromboelastography (TEG) and throm-boelastometry, measure changes in viscoelastic properties of whole-blood clotting [1]. Since they allow rapid assessment of global hemostatic function, they have been used to guide transfusion therapy during liver transplantation and cardiac bypass surgery. In the past, the tests required non-citrated whole blood used within 5 minutes after collecting. Today, citrated whole blood can be used instead, which allows them to detect many clinical bleeding disorders in a central laboratory [2].
TEG measures different parameters of clot formation and clot lysis: reaction time (R), the time to initial fibrin formation, reflects the circulating levels of coagulation factors; time to clot formation (K); alpha angle (α), which shows the fibrinogen level; maximum amplitude (MA), reflects the combined levels of platelets and fibrinogen; percentage of fibrinolysis at 30 minutes after MA (LY30); and coagulation index (CI), a linear combination of R, K, α, and MA [3]. To evaluate blood coagulation, we need to establish reference ranges for TEG parameters and understand the factors that affect them. For instance, several studies have reported abnormal or normal TEG parameter values in patients with certain coagulation factor deficiencies [4-6]. Furthermore, demonstrating whether TEG parameters correlate with levels of coagulation and anticoagulation factors by simultaneous measuring has never been shown.
Here, we established normal reference ranges for six TEG parameters and investigated their correlation with different coagulation and anticoagulation factors. In addition, we assessed whether TEG detects coagulation factor deficiencies.
In total, 109 healthy volunteers and four patients with coagulation factor deficiencies participated in the study after approval from the Institutional Review Board of Seoul National University Hospital. All individuals were Korean. “Healthy” was defined as free from acute illness and without hospitalization for any illness during the previous 6 months. We excluded subjects with any bleeding or thrombotic disorders, recent illnesses, and those taking anticoagulants.
The mean age of the volunteers was 36 years (21–62 years). Blood samples were collected by venipuncture into commercially available tubes containing 0.109 mol/L sodium citrate (Becton Dickinson, San Jose, CA, USA). For thrombin generation assay and other coagulation tests, plasma was separated by centrifugation of whole blood at 1,550×g for 15 minutes within 2 hours after blood sampling. Plasma aliquots were stored at −80°C.
According to the manufacturer’s instructions, 1 mL of the citrated whole blood was pipetted into a vial containing buffered stabilizers and kaolin (Haemonetics, Niles, IL, USA). After five gentle inversions, 340 μL of kaolin-activated blood was pipetted into a disposable cup containing calcium chloride and analyzed with the TEG 5000 Hemostasis Analyzer System (Haemonetics, Niles, IL, USA) for 50 minutes. Six TEG parameters were recorded: time to initial fibrin formation (R), time to clot formation (K), alpha angle (α), maximum amplitude (MA), percentage of fibrinolysis at 30 minutes after MA (LY30), and coagulation index (CI).
To measure the functional fibrinogen level (FLEV), 0.5 mL of the citrated whole blood was pipetted into a functional fibrinogen reagent vial (Haemonetics Corporation, Niles, IL, USA), containing a tissue factor and platelet inhibitor. After five gentle inversions, the blood was treated with the procedure described above.
Tissue factor–triggered thrombin generation in platelet-poor plasma (PPP) was analyzed using the calibrated automated thrombogram method (Thrombinoscope, Maastricht, Netherlands). Wells of a round-bottomed 96 well plate containing the tissue factor at a final concentration of 1 pmol/L with phospholipids or thrombin calibrators was filled with 20 μL of the PPP reagent and 80 μL of the test plasma. After adding 20 μL of a fluorogenic substrate to HEPES buffer containing calcium chloride, fluorescence was measured in the Fluoroskan Ascent fluorometer (Thermo Labsystems, Helsinki, Finland) and thrombin generation curves were calculated with the Thrombinoscope software (Thrombinoscope, Maastricht, Netherlands). Thrombin generation curves were analyzed using parameters that represent the initiation, propagation, and termination phases of thrombin generation (lag time, endogenous thrombin potential [ETP], and peak thrombin height).
Coagulation tests were conducted on an automated coagulation analyzer (ACL TOP; Beckman Coulter, Fullerton, CA, USA): prothrombin time (PT), activated partial thromboplastin time (aPTT), fibrinogen, coagulation and anticoagulation factor assays. PT was measured using the HemosIL RecombiPlasTin reagent (Instrumentation Laboratory, Milan, Italy) and aPTT using the SynthASil reagent (Instrumentation Laboratory). Fibrinogen activity was measured using the HemosIL Fibrinogen-C XL reagent (Instrumentation Laboratory) based on the Clauss method [7]. Coagulation factors FII, FV, FVII, and FX were quantified using a PT-based clotting assay with the HemosIL RecombiPlasTin reagent and factors FVIII, FIX, FXI, and FXII were quantified with the aPTT-based clotting assay using the SynthASil reagent. Protein C, protein S, and plasminogen activity were determined with chromogenic assays (HemosIL Antithrombin, Protein C, or Plasminogen, Instrumentation Laboratory) and an immunoassay (HemosIL Free Protein S, Instrumentation Laboratory).
The reference ranges were determined as 90% reference intervals of data from healthy volunteers according to the Clinical and Laboratory Standards Institute (EP28-A3c)[8]. The difference between genders was evaluated using the Mann–Whitney U test. Correlations of TEG parameters with other coagulation data were expressed as Spearman’s coefficients. All analyses were conducted using SPSS version 12.0 (SPSS Inc., Chicago, IL, USA). The probability of less than 0.05 (P<0.05) was considered significant.
The tested demographic characteristics of the 109 healthy participants are summarized in Table 1, while the reference ranges for six analyzed TEG parameters are in Table 2. Overall, the K values were lower in females than males, whereas α, MA, and CI values were higher. FLEV was also higher in females than males.
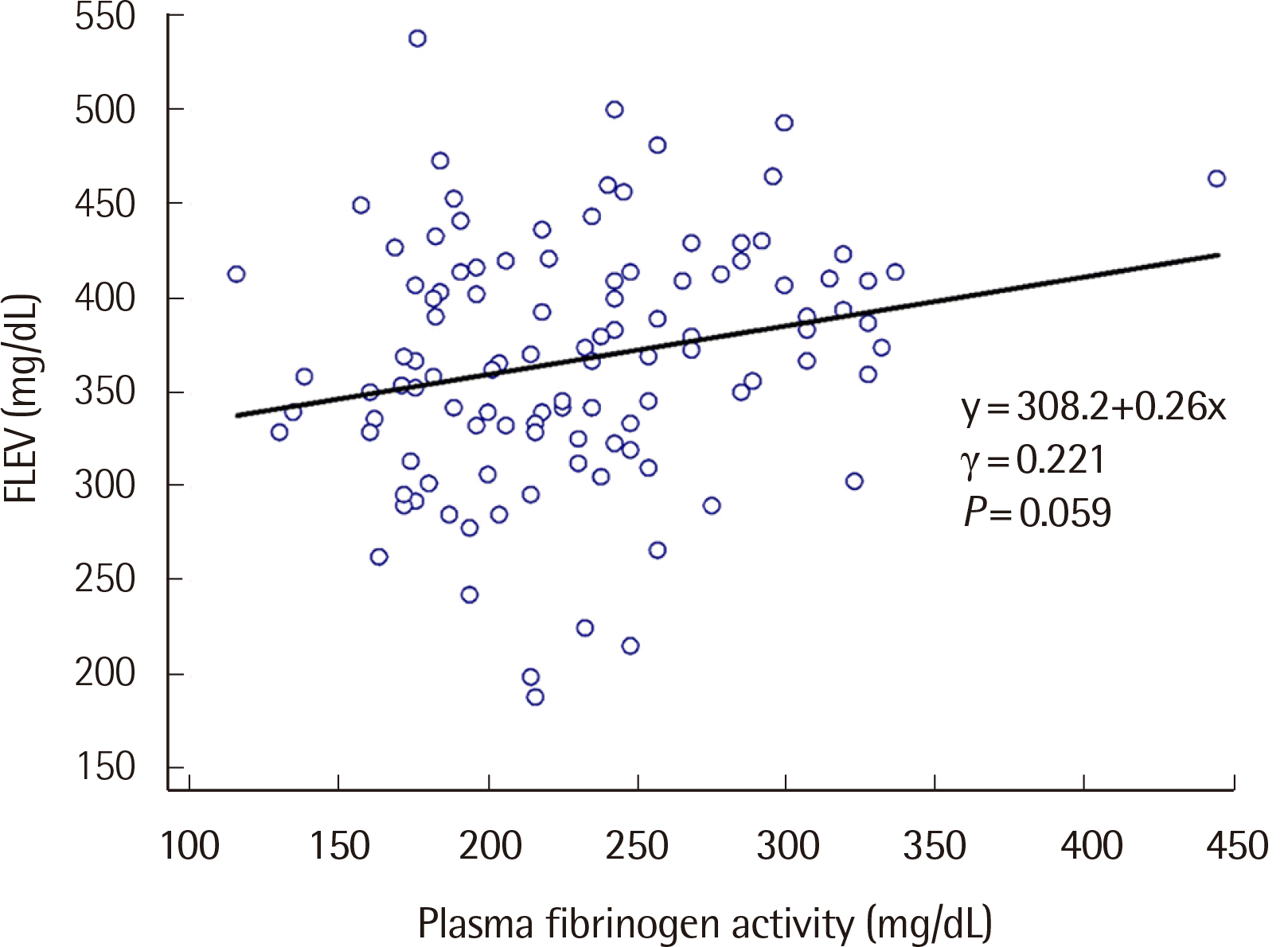
To investigate whether blood coagulation factors affect TEG parameters, we quantified all coagulation and anticoagulation factors in the healthy participants and performed a TGA assay. Because of significant multicollinearity among coagulation and anticoagulation factors, multiple regression analysis was not possible. Thus, simple linear correlations between the factors and TEG parameters were performed instead (Table 3). Some TEG parameters exhibited only negative correlation with the factors. For example, R negatively correlated with factors V (γ=−0.192) and XII (γ=−0.208) and K with factor XII (γ=−0.252). Alpha angle (α) showed positive correlation with factor XII (γ=0.218) and negative with protein S (γ=−0.195). Furthermore, MA and CI showed only positive correlation where MA correlated with fibrinogen (γ=0.337), factor VIII (γ=0.241), factor XII (γ=0.261), protein C (γ=0.215), plasminogen (γ=0.201), and peak thrombin (γ=0.196) and CI with factors VIII (γ=0.206) and XII (γ=0.269). The only TEG parameter that showed no correlation with coagulation factors or plasminogen was LY30. A moderate correlation was also observed between FLEV and plasma fibrinogen activity (γ=0.221) (Fig. 1).
To assess whether the TEG parameters detect coagulation factor deficiencies, we performed the TEG assay in four patients with known coagulation factor deficiencies (Table 4). The patient with factor V deficiency (case 1), who showed a factor V activity of 17%, also showed abnormal R, PT, and aPTT values. Two patients with factor VII deficiency, who had a factor VII activity of 5% (case 2) and 27% (case 3), respectively, exhibited normal R values despite abnormal PT. The patient with fibrinogen deficiency (case 4), who had a fibrinogen level of 119 mg/dL, showed normal MA (43.3) and FLEV (228.1), although they were close to the lower limits of the reference ranges (MA, 42.7–73.4; FLEV, 211.2–494.5).
TEG tests the efficiency of whole-blood coagulation by measuring parameters that reflect different phases of hemostasis [2]. It was unknown whether coagulation and anticoagulation factors affect TEG parameters. By measuring coagulation and anticoagulation factor levels in a healthy population, this study was the first to show their correlation with TEG parameters.
Our study demonstrated that R correlates with factors V and XII but not with other factors. Although we could not show associations between the factors and TEG parameters with multiple regression analysis due to the high multicollinearity, we demonstrated significant associations with simple linear correlation analysis. To confirm the correlation between the R time and the two factors, we evaluated four patients with known factor deficiencies. The patient with factor V deficiency (case 1) showed an increased R value; therefore, R is sensitive to factor V deficiency. This finding also verifies the significant correlation between R and factor V. The patients with factor VII deficiency (cases 2 and 3) did not show increased R values; thus, R is insensitive to factor VII deficiency. Consistent with this result, Tran et al. [4] reported that TEG is not suitable to detect this deficiency. The R time has also been reported as inferior to PT at identifying vitamin K–dependent coagulation factor deficiency [6].
MA represents the maximum strength of the final hemostatic clot and generally reflects fibrinogen activity, platelet count, and platelet function [9]. Our study found a significant positive correlation between fibrinogen activity and MA. Although our case 4 patient with fibrinogen deficiency had a normal MA value (43.3), it was close to the lower limit of the reference range for MA (42.7). Platelet count mainly affects MA [9]. Since the count was unavailable for our study, we cannot exclude it, and we can consider it obscured the fibrinogen effect on MA. Furthermore, MA significantly correlated with factor VIII, protein C, and plasminogen. We previously reported that measured fibrinogen activity significantly correlates with factor VIII and protein C levels in a healthy population [10, 11]. Therefore, persons with high fibrinogen levels likely also have high factor VIII and protein C levels, which may mimic the correlation between these factors and MA.
Although K and α both reflect fibrinogen activity and platelet count, we found no significant correlation between them. Thus, more studies are required to confirm whether these factors affect K and α parameters. Interestingly, factor XII significantly correlated with various parameters: R, K, α, MA, and CI. Since we used kaolin as a coagulation activator in our TEG assay, the speed and strength of hemostatic clot formation in a TEG cup were likely sensitive to the activated factor XII levels.
The relative distribution of reference ranges between male and female participants showed a similar pattern in previous reports [12-14]. As implied by the TEG parameters, women showed a more hypercoagulable profile than men, although there were no significant differences in PT and aPTT between them. Indeed, the incidence of thromboembolism is higher in women than men due to estrogen exposure [15, 16]. TEG parameters thus seem more sensitive indicators of hypercoagulability than routine coagulation tests, such as PT and aPTT.
TEG can measure FLEV with a tissue factor that activates the extrinsic pathway and platelet inhibitors, hence eliminating the platelet effect on MA [17]. In our study, FLEV moderately correlated with plasma fibrinogen activity (r=0.221), unlike in another study where the correlation was strong (r=0.933) [17]. This study assessed a population of trauma patients that showed a wide range of fibrinogen levels. In contrast, our study evaluated a population of healthy subjects that demonstrated a narrow range of fibrinogen levels within the reference range. Therefore, the population type likely accounts for the difference in fibrinogen levels between the two studies.
Our study has several potential limitations. First, and as mentioned previously, although platelet count is the main contributor to MA, we could not measure the platelet count in the healthy participants. Second, because the coagulation factor deficiencies are rare, we had only four patients to evaluate the detection ability of the TEG parameters. Third, we could not apply the strict definition of heathy adults to establish a normal reference range for TEG parameters because we tested less than 120 healthy adults needed for a reference range of nonparametric 90% reference interval. Nonetheless, since this is the only Korean study showing the reference ranges for TEG parameters in healthy Korean adults, our results can be used as a preliminary reference.
In summary, this study demonstrated that TEG parameters correlated with the levels of several coagulation factors. The R value significantly correlated with factor V and the MA value with fibrinogen. Of note, factor XII significantly correlated with R, K, α, MA, and CI. We expect that our findings will help to interpret the TEG parameters in a normal population. To further evaluate the diagnostic ability of TEG in detecting specific coagulation factor deficiencies, future studies on large cohorts are necessary.
Acknowledgement
This work was supported by the National Research Foundation of Korea (NRF) grant (2021R1A2C1006302), funded by the Korean government (MSIT).
REFERENCES
1. Chitlur M. 2012; Challenges in the laboratory analyses of bleeding disorders. Thromb Res. 130:1–6. DOI: 10.1016/j.thromres.2012.03.011. PMID: 22483776.

2. Othman M, Kaur H. 2017; Thromboelastography (TEG). Methods Mol Biol. 1646:533–43. DOI: 10.1007/978-1-4939-7196-1_39. PMID: 28804853.

3. Müller MC, Meijers JC, Vroom MB, Juffermans NP. 2014; Utility of thromboelastography and/or thromboelastometry in adults with sepsis: a systematic review. Crit Care. 18:R30. DOI: 10.1186/cc13721. PMID: 24512650. PMCID: PMC4056353.

4. Tran HT, Tjønnfjord GE, Holme PA. 2014; Use of thromboelastography and thrombin generation assay to predict clinical phenotype in patients with severe FVII deficiency. Haemophilia. 20:141–6. DOI: 10.1111/hae.12256. PMID: 23992369.

5. Spiezia L, Radu C, Campello E, Bulato C, Bertini D, Barillari G, et al. 2012; Whole blood rotation thromboelastometry (ROTEM®) in nine severe factor V deficient patients and evaluation of the role of intraplatelets factor V. Haemophilia. 18:463–8. DOI: 10.1111/j.1365-2516.2011.02710.x. PMID: 22176523.
6. Nascimento B, Al Mahoos M, Callum J, Capone A, Pacher J, Tien H, et al. 2012; Vitamin K-dependent coagulation factor deficiency in trauma: a comparative analysis between international normalized ratio and thromboelastography. Transfusion. 52:7–13. DOI: 10.1111/j.1537-2995.2011.03237.x. PMID: 21745216.

7. Lowe GD, Rumley A, Mackie IJ. 2004; Plasma fibrinogen. Ann Clin Biochem. 41:430–40. DOI: 10.1258/0004563042466884. PMID: 15588432.

8. Clinical, Laboratory Standards Institute. 2010. Defining, establishing, and verifying reference intervals in the clinical laboratory; approved guideline-Third edition. CLSI document EP28-A3c. Wayne, PA: Clinical and Laboratory Standards Institute.
9. Chen A, Teruya J. 2009; Global hemostasis testing thromboelastography: old technology, new applications. Clin Lab Med. 29:391–407. DOI: 10.1016/j.cll.2009.04.003. PMID: 19665684.

10. Kim SY, Kim JE, Kim HK, Kim I, Yoon SS, Park S. 2013; Influence of coagulation and anticoagulant factors on global coagulation assays in healthy adults. Am J Clin Pathol. 139:370–9. DOI: 10.1309/AJCPC5C4AGFRDKMX. PMID: 23429374.

11. Choi Q, Kim JE, Kim SY, Han KS, Kim HK. 2015; Influence of ABO type on global coagulation assay results: effect of coagulation factor VIII. Clin Chem Lab Med. 53:1425–32. DOI: 10.1515/cclm-2014-0909. PMID: 25503670.

12. Macafee B, Campbell JP, Ashpole K, Cox M, Matthey F, Acton L, et al. 2012; Reference ranges for thromboelastography (TEG®) and traditional coagulation tests in term parturients undergoing caesarean section under spinal anaesthesia*. Anaesthesia. 67:741–7. DOI: 10.1111/j.1365-2044.2012.07101.x. PMID: 22486761.

13. Scarpelini S, Rhind SG, Nascimento B, Tien H, Shek PN, Peng HT, et al. 2009; Normal range values for thromboelastography in healthy adult volunteers. Braz J Med Biol Res. 42:1210–7. DOI: 10.1590/S0100-879X2009001200015. PMID: 19882085.

14. Polak F, Kolnikova I, Lips M, Parizek A, Blaha J, Stritesky M. 2011; New recommendations for thromboelastography reference ranges for pregnant women. Thromb Res. 128:e14–7. DOI: 10.1016/j.thromres.2011.04.007. PMID: 21543108.

15. Bleker SM, Coppens M, Middeldorp S. 2014; Sex, thrombosis and inherited thrombophilia. Blood Rev. 28:123–33. DOI: 10.1016/j.blre.2014.03.005. PMID: 24768093.

16. Moores L, Bilello KL, Murin S. 2004; Sex and gender issues and venous thromboembolism. Clin Chest Med. 25:281–97. DOI: 10.1016/j.ccm.2004.01.013. PMID: 15099889.

17. Harr JN, Moore EE, Ghasabyan A, Chin TL, Sauaia A, Banerjee A, et al. 2013; Functional fibrinogen assay indicates that fibrinogen is critical in correcting abnormal clot strength following trauma. Shock. 39:45–9. DOI: 10.1097/SHK.0b013e3182787122. PMID: 23247121. PMCID: PMC3529156.

Table 1
Evaluated demographic characteristics with quantified blood coagulation factors and thrombin generation in healthy participants
| Total population (N=109) | Males (N=44) | Females (N=65) | |
|---|---|---|---|
|
|
|
|
|
| Mean±SD | Mean±SD | Mean±SD | |
| Age | 35.8 ± 8.8 | 34.4 ± 8.6 | 37.9 ± 8.8 |
| Height (kg/m2) | 22.1 ± 2.8 | 21.2 ± 2.4 | 23.3 ± 2.8* |
| Fibrinogen | 230.0 ± 55.3 | 230.0 ± 55.3 | 219.9 ± 51.6 |
| FII | 104.2 ± 14.6 | 104.2 ± 14.6 | 105.2 ± 15.3 |
| FV | 99.9 ± 22.2 | 99.9 ± 22.2 | 99.5 ± 23.9 |
| FVII | 98.2 ± 21.5 | 98.2 ± 21.5 | 100.3 ± 20.2 |
| FVIII | 82.3 ± 23.6 | 82.3 ± 23.6 | 80.8 ± 21.0 |
| FIX | 104.9 ± 20.4 | 104.9 ± 20.4 | 110.3 ± 22.3* |
| FX | 95.9 ± 21.6 | 95.9 ± 21.6 | 98.3 ± 21.7 |
| FXI | 86.4 ± 15.6 | 86.4 ± 15.6 | 89.1 ± 14.5 |
| FXII | 80.0 ± 23.9 | 80.0 ± 23.9 | 82.0 ± 23.5 |
| Antithrombin | 93.0 ± 11.0 | 93.0 ± 11.0 | 95.5 ± 10.3* |
| Protein C | 105.7 ± 18.2 | 105.7 ± 18.2 | 107.0 ± 19.6 |
| Protein S | 91.9 ± 19.3 | 91.9 ± 19.3 | 103.0 ± 17.8* |
| Plasminogen | 92.5 ± 13.0 | 92.5 ± 13.0 | 91.1 ± 13.7 |
| ETP | 641.1 ± 214.9 | 641.1 ± 214.9 | 646.8 ± 242.4 |
| Lag time | 3.8 ± 0.7 | 3.8 ± 0.7 | 3.8 ± 0.8 |
| Peak thrombin | 80.5 ± 45.7 | 80.5 ± 45.7 | 85.9 ± 45.7 |
Table 2
Reference ranges for thromboelastography parameters in healthy participants
| Total population (N=109) | Males (N=44) | Females (N=65) | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Mean±SD | Reference range* | Mean±SD | Reference range* | Mean±SD | Reference range* | |
| R (min) | 5.7 ± 1.4 | 3.5–8.7 | 6.0 ± 1.4 | 3.8–8.8 | 5.6 ± 1.3 | 3.3–8.6 |
| K (min) | 1.8 ± 0.6 | 1.0–2.8 | 2.1 ± 0.5 | 1.1–3.0 | 1.7 ± 0.6 | 0.9–2.8† |
| α (degree) | 64.0 ± 6.8 | 50.6–73.6 | 61.1 ± 6.3 | 50.0–71.8 | 66.0 ± 6.5 | 53.8–74.9† |
| MA (mm) | 59.4 ± 7.3 | 45.8–71.1 | 56.5 ± 7.1 | 42.5–68.5 | 61.4 ± 6.8 | 48.8–72.6† |
| LY30 (%) | 1.0 ± 1.7 | 0.0–5.3 | 0.8 ± 1.4 | 0.0–4.1 | 1.1 ± 1.9 | 0.0–5.9 |
| CI | –0.1 ± 2.1 | -3.6–3.0 | -0.9± 1.9 | -4.4–2.6 | 0.4 ± 2.1† | -3.4–3.3† |
| FLEV (mg/dL) | 367.9 ± 64.8 | 252.5–469.0 | 339.6 ± 63 | 228.9–462.6 | 387± 59.1 | 286.3–478.8† |
| PT (s) | 11.2 ± 0.8 | 10.0–13.1 | 11.1 ± 0.8 | 10.0–12.6 | 11.2 ± 0.9 | 10.0–13.2 |
| aPTT (s) | 32.4 ± 2.8 | 26.9–36.8 | 32.4 ± 2.7 | 27.3–37.2 | 32.3 ± 2.9 | 26.4–36.7 |
Table 3
Correlation of thromboelastography parameters with coagulation and anticoagulation factors
| R | K | α | MA | LY30 | CI | FLEV | |
|---|---|---|---|---|---|---|---|
| Age | -0.090 | -0.062 | 0.075 | 0.034 | -0.098 | 0.083 | -0.068 |
| Fibrinogen | 0.061 | -0.135 | 0.144 | 0.337† | 0.075 | 0.154 | 0.221* |
| FII | -0.100 | -0.050 | 0.040 | 0.075 | -0.022 | 0.084 | 0.007 |
| FV | -0.192* | -0.101 | 0.104 | 0.036 | 0.171 | 0.127 | 0.003 |
| FVII | -0.036 | -0.032 | -0.007 | 0.095 | 0.027 | 0.052 | -0.031 |
| FVIII | -0.155 | -0.156 | 0.133 | 0.241* | -0.035 | 0.206* | 0.173 |
| FIX | -0.071 | -0.098 | 0.056 | 0.112 | -0.106 | 0.095 | -0.069 |
| FX | -0.046 | -0.096 | 0.075 | 0.032 | -0.035 | 0.057 | -0.091 |
| FXI | -0.054 | -0.017 | 0.014 | 0.070 | 0.044 | 0.055 | 0.016 |
| FXII | -0.208* | -0.252† | 0.218* | 0.261† | -0.094 | 0.269† | 0.121 |
| Antithrombin | -0.007 | 0.079 | -0.095 | -0.071 | 0.040 | -0.058 | -0.093 |
| Protein C | -0.090 | -0.172 | 0.125 | 0.215* | -0.142 | 0.169 | 0.058 |
| Protein S | 0.076 | 0.168 | -0.195* | -0.166 | 0.083 | -0.162 | -0.110 |
| Plasminogen | 0.059 | -0.150 | 0.103 | 0.201* | 0.008 | 0.093 | 0.151 |
| ETP | 0.041 | -0.112 | 0.116 | 0.162 | -0.050 | 0.087 | 0.023 |
| Lag time | -0.051 | -0.047 | 0.006 | 0.009 | 0.056 | 0.026 | 0.114 |
| Peak thrombin | -0.005 | -0.111 | 0.132 | 0.196* | -0.152 | 0.136 | 0.041 |
Table 4
Results of thromboelastography and other coagulation tests in four patients with coagulation factor deficiencies
| Case | Diagnosis | Deficient factor level | Thromboelastography | Coagulation tests | |||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| R (min) | K (min) | α (deg) | MA (mm) | FLEV (mg/dL) | PT (s) | aPTT (s) | |||
| 1 | FV deficiency | FV: 17.0% | 9.2* | 1.2 | 68.5 | 62.7 | 390.5 | 14.7* | 55.1* |
| 2 | FVII deficiency | FVII: 5.0% | 7.7 | 2.0 | 61.8 | 66.2 | 275.5 | 163.5* | 34.9 |
| 3 | FVII deficiency | FVII: 27.0% | 6.8 | 2.0 | 62.4 | 65.0 | 302.9 | 16.5* | 30.7 |
| 4 | Fibrinogen deficiency | Fibrinogen: 119 mg/dL | 6.0 | 1.7 | 62.7 | 43.3 | 228.1 | 11.7 | 33.8 |
*The values are out of the reference ranges shown in Table 2.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download