INTRODUCTION
MATERIALS AND METHODS
Participants
Study design
Data collection
Determination of serum MMP-3 levels
Statistical analysis
RESULTS
Baseline characteristics of the participants
Table 1
| Characteristics |
Total (n=95) |
Methotrexate group (n=48) | Tocilizumab group (n=47) | p-value |
|---|---|---|---|---|
| Age (yr) | 52.3±11.3 | 52.0±12.24 | 52.6±10.4 | 0.806 |
| Female | 84 (88.4) | 42 (87.5) | 42 (89.4) | 0.777 |
| Disease duration (yr) | 9.87±7.5 | 8.9±7.2 | 10.8±7.8 | 0.220 |
| Smoking history | 5 (5.3) | 3 (6.3) | 2 (4.3) | 1.000 |
| Weight (kg) | 56.46±9.2 | 57.7±10.2 | 55.2±8.0 | 0.172 |
| TJC (66 assessed) | 22.3±13.1 | 22.8±14.0 | 21.8±12.1 | 0.722 |
| SJC (68 assessed) | 11.1±7.9 | 11.9±10.0 | 10.3±4.9 | 0.319 |
| Patient’s pain VAS (0~100) | 59.7±22.4 | 60.7±21.8 | 58.6±23.1 | 0.654 |
| Patient’s global VAS (0~100) | 65.0±22.2 | 65.9±20.0 | 64.0±24.4 | 0.673 |
| Physician’s global VAS (0~100) | 63.2±16.9 | 63.0±18.1 | 63.4±15.7 | 0.917 |
| HAQ-DI | 1.32±0.67 | 1.4±0.6 | 1.3±0.7 | 0.418 |
| Laboratory findings | ||||
| RF positivity | 58 (61.1) | 24 (50.0) | 34 (72.3) | 0.0256 |
| RF titer (U/mL) (n=54)* | 102.27±156.37 | 164±217 | 162±191 | 0.246 |
| ESR (mm/hr) | 52.3±26.2 | 52.5±27.1 | 52.2±25.6 | 0.958 |
| CRP (mg/dL) | 2.9±2.6 | 3.0±2.7 | 2.7±2.5 | 0.656 |
| MMP-3 (ng/mL) (n=90) | 290.0±206.4 | 277.9±189.7 | 302.1±223.4 | 0.581 |
| IL-6 (pg/mL) | 8.7±5.1 | 8.9±4.9 | 8.6±5.4 | 0.818 |
| sIL-6R (ng/mL) | 130.0±68.7 | 127.4±68.9 | 132.7±69.1 | 0.708 |
| DAS28-ESR | 6.9±0.9 | 6.8±1.0 | 6.9±0.9 | 0.972 |
| Low disease activity | 0 (0.0) | |||
| Moderate disease activity | 0 (0.0) | |||
| High disease activity | 95 (100.0) | |||
| DAS28-CRP (choice one) | 6.2±1.0 | 6.2±1.0 | 6.2±1.0 | 0.762 |
| Low disease activity | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Moderate disease activity | 13 (13.7) | 6 (12.5) | 7 (14.9) | 0.734 |
| High disease activity | 82 (86.3) | 42 (87.5) | 40 (85.1) | |
| SDAI | 45.3±14.7 | 45.7±15.6 | 44.8±13.9 | 0.754 |
| CDAI | 42.4±13.6 | 42.8±15.0 | 42.0±12.3 | 0.800 |
| DMARDs | ||||
| MTX alone | 54 (56.8) | 26 (54.2) | 28 (59.6) | 0.595 |
| MTX dose (mg/wk) | 14.18±3.21 | 13.6±3.4 | 14.5±3.0 | 0.314 |
| Hydroxychloroquine | 32 (33.7) | 19 (39.6) | 13 (27.7) | 0.219 |
| Sulfasalazine | 9 (9.5) | 3 (6.3) | 6 (12.8) | 0.278 |
| Oral glucocorticoid | 84 (88.4) | 45 (93.8) | 39 (83.0) | 0.101 |
Values are presented as mean±standard deviation or number (%). CDAI: Clinical Disease Activity Index, CRP: C-reactive protein, DAS28: 28-joint Disease Activity Score, DMARD: disease-modifying antirheumatic drug, ESR: erythrocyte sedimentation rate, HAQ-DI: Health Assessment Questionnaire disability index, IL-6: interleukin-6, MMP-3: metalloproteinase-3, MTX: methotrexate, RF: rheumatoid factor, SDAI: Simplified Disease Activity Index, sIL-6R: soluble IL-6 receptor, SJC: swollen joint count, TJC: tender joint count, VAS: visual analog scale. *Based on the calculation from the patients with positive RF (≥15 U/mL).
Changes in serum MMP-3 levels and disease activity indices in RA patients during the 24 weeks
Fig. 1
Correlations between MMP-3 and other biomarkers
Fig. 2
Table 2
CDAI: Clinical Disease Activity Index, CRP: C-reactive protein, DAS28: 28-joint Disease Activity Score, ESR: erythrocyte sedimentation rate, IL-6: interleukin-6, MMP-3: metalloproteinase-3, PGA: Physician’s global assessment, PtGA: Patient’s global assessment, SDAI: Simplified Disease Activity Index, sIL-6R: soluble IL-6 receptor, SJC: swollen joint count, TJC: tender joint count. Correlation analysis were derived from measurements taken from 95 patients with RA at baseline and 12, 20, and 24 weeks. Pearson’s correlation coefficient (r) and p-value levels are decribed.
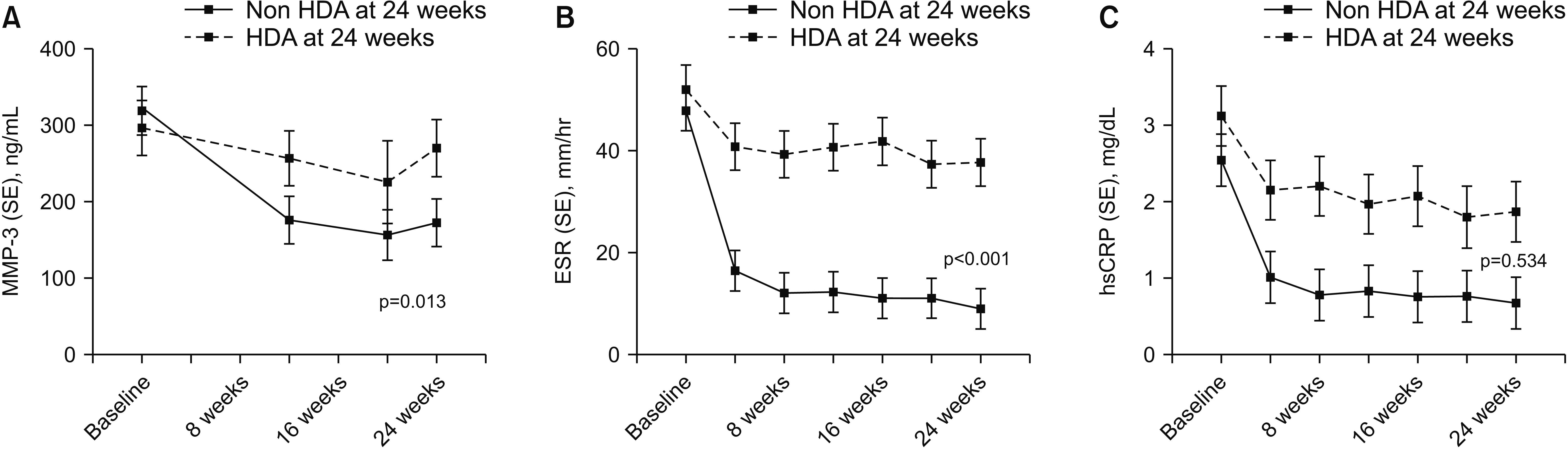
Comparison of changes in serum levels of MMP-3 and acute phase reactants during the treatment period according to disease activity at 24 weeks
Fig. 3




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download