INTRODUCTION
Tuberculosis (TB) is a serious infectious disease caused by
Mycobacterium tuberculosis that has become a global public health problem. It usually affects the lungs, but it can affect any part of the body. Extrapulmonary TB is defined as the occurrence of TB at sites other than the lungs, and it accounts for approximately 15% of all active TB infections [
1].
Bone and joint TB, which accounts for approximately 10 to 11 percent of extrapulmonary TB, occurs as a result of the seeding of organisms in bone and/or synovial tissue [
2]. TB arthritis, a form of skeletal TB affecting peripheral joints, is generally a monoarticular disease that typically involves large weight-bearing joints, such as the hip and knee [
3]. However, its diagnosis is often delayed or inaccurate due to a lack of awareness and the disease’s ability to mimic other inflammatory arthritides, such as rheumatoid arthritis (RA) and crystal-induced arthropathy. Poncet’s disease (PD), a reactive polyarthritis in the presence of active TB, may also arise without bacterial involvement of the joint [
4]. TB arthritis, PD, and RA may have similar clinical characteristics, including a chronic course with swollen joints and radiologic findings, rendering differential diagnosis difficult [
5].
RA is known to be associated with an increased risk of serious infection [
6], which appears to be greater in patients with higher RA disease activity than in those with low disease activity or remission [
7]. In addition, recent developments in the treatment of RA, particularly biologic agents such as tumor necrosis factor (TNF)-α inhibitors, are associated with the reactivation of TB [
8,
9]. Accordingly, detection of inflammatory arthritis associated with TB is notably important during the clinical course of RA. However, only few studies have focused on a comprehensive review of inflammatory arthritides that occur with active TB infection. Herein, we reviewed cases of active TB infection associated with joint pain to ascertain the clinical manifestations of inflammatory arthritis accompanying TB and to aid in the differential diagnosis of RA.
Go to :

RESULTS
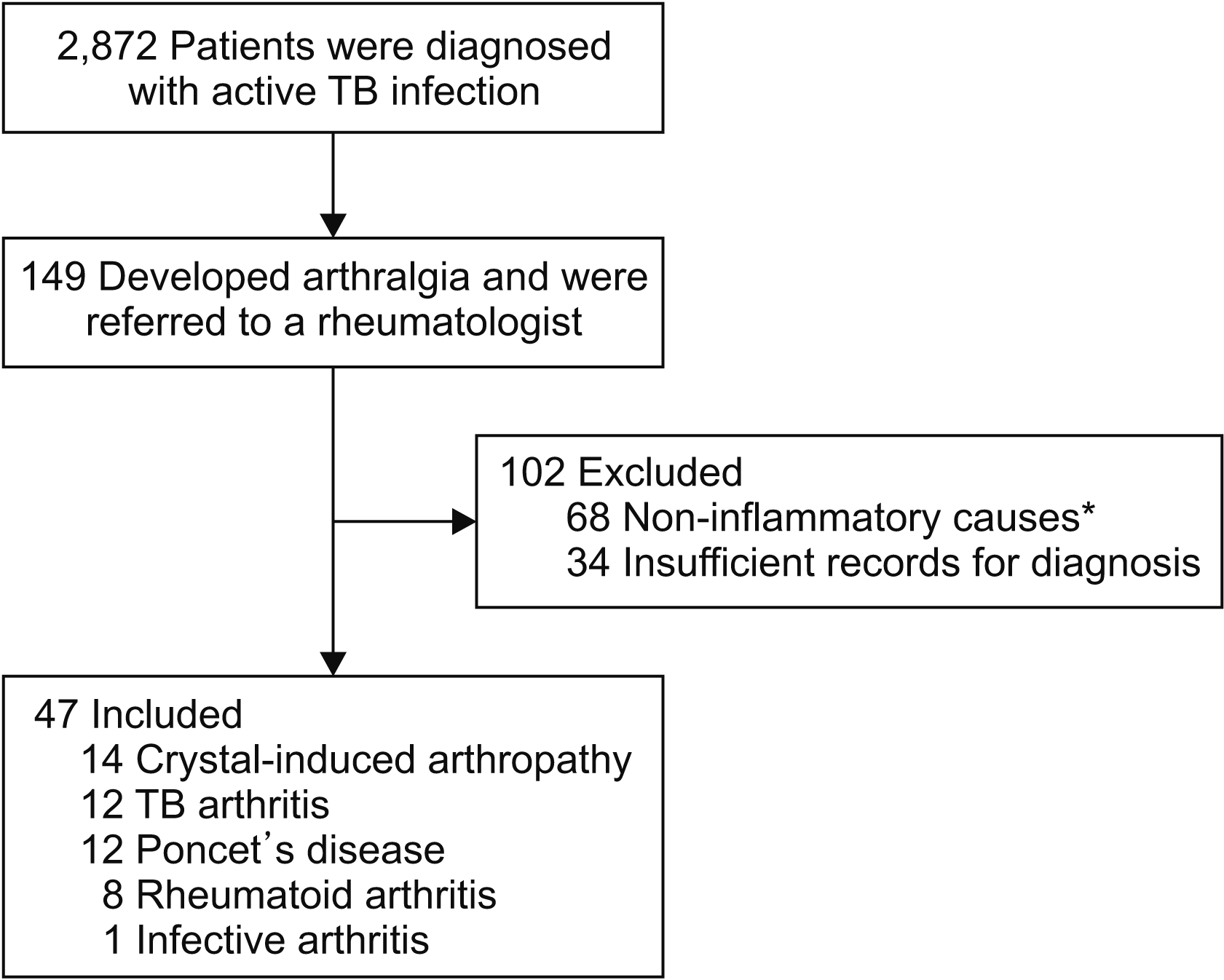
Among 2,872 patients with active TB infection, 47 had inflammatory arthropathy: 14 had crystal-induced arthropathy; 12, TB arthritis; 12, PD; 8, RA; and 1, septic arthritis. Clinical characteristics of each inflammatory arthritis are summarized in
Table 1.
Table 1
Clinical characteristics of inflammatory arthropathy in 47 patients with active TB infection
|
Characteristics |
TB arthritis
(n=12) |
Poncet's disease
(n=12) |
Rheumatoid arthritis
(n=8) |
Crystal-induced arthropathy
(n=14) |
Infective arthritis
(n=1) |
|
Age (yr) |
61 (41~82) |
63 (35~85) |
68 (59~88) |
72 (46~89) |
75 |
|
Sex, male |
8 (66.7) |
7 (58.3) |
4 (50.0) |
11 (78.6) |
0 |
|
Extrapulmonary TB |
6 (50.0) |
4 (33.3) |
0 |
2 (14.3) |
1 |
|
Number of affected joints*
|
|
|
|
|
|
|
Monoarthritis |
10 |
0 |
0 |
10 |
1 |
|
Oligoarthritis |
2 |
3 |
5 |
4 |
0 |
|
Polyarthritis |
0 |
9 |
3 |
0 |
0 |
|
Distribution of affected joints |
|
|
|
|
|
|
Large-sized joints |
8 |
0 |
0 |
4 |
1 |
|
Small-to-medium-sized joints |
2 |
2 |
5 |
8 |
0 |
|
Mixed†
|
2 |
10 |
3 |
2 |
0 |
|
ESR (mm/hr) |
72 (48~120) |
68 (39~110) |
82 (55~108) |
47 (36~76) |
92 |
|
CRP (mg/dL) |
5.6 (1.2~8.9) |
5.9 (1.4~13.5) |
7.4 (1.8~18.3) |
7.8 (1.6~19.5) |
31.7 |
|
Arthritis development predating the diagnosis of TB |
11 (91.7) |
9 (75.0) |
8 (100) |
1 (7.1) |
0 |
|
Duration of arthritis symptoms after anti-TB treatment (d) |
90 (45~120) |
56 (14~90) |
120 (30~180) |
12 (5~21) |
60 |

In TB arthritis, the weight-bearing joints such as the hip, knee, and ankle were more commonly involved than the elbow and wrist joints (66.7% vs. 30%). When compared to TB arthritis, PD demonstrated a significantly higher proportion of polyarthritis (75% vs. 0%) and involved both large and small-to-medium-sized joints (83.3% vs. 16.7%). Arthritis symptoms antedated the diagnosis of TB infection in both TB arthritis and PD (91.7% and 75%, respectively). The duration of arthritis symptoms after anti-TB treatment was significantly shorter in patients with PD (56 days vs. 90 days, p=0.028). Age, sex, primary site of TB infection (pulmonary vs. extrapulmonary TB), and serum ESR and CRP levels were not statistically different between TB arthritis and PD. One patient with TB arthritis and one with PD had underlying RA prior to the onset of active TB infection. The initial manifestations were mono- or oligoarthritis mimicking an RA flare-up without respiratory symptoms. Despite screening for latent TB infection, pulmonary TB occurred within 3 months after treatment with a TNF-α blocker.
Among the total number of active TB patients in the present study, the prevalence of RA was 1.08% (31/2,872), and its median duration was 5.8 (IQR, 2.5~14.2) years. The median prednisolone equivalent daily dose was 7.5 (IQR, 0~25) mg at the diagnosis of active TB infection. Of the 31 RA patients, 8 had a flare-up of arthritis during active TB infections. Although all of them had been diagnosed with pulmonary TB, constitutional symptoms such as fever and chills or respiratory symptoms were absent at the onset of RA flares. Consequently, it took a median of 3.4 (IQR, 1.25~5) months from the onset of arthritis symptoms to diagnose active TB infection. RA flares during active TB infection were oligo- or polyarthritis, such as PD (
Table 1). There was no significant difference in ESR or CRP levels between patients with RA flares and those with PD. However, the RA flares involved only small-to-medium-sized joints rather than a mixed distribution when compared to PD (62.5% vs. 16.7%, p=0.035). In PD, a significantly higher number of patients had fever at disease onset (OR, 24; 95% CI, 1.78~248.1), showed a good response to non-steroidal anti-inflammatory drugs (NSAIDs) alone (OR, 35; 95% CI, 2.63~465.4), and had relief from arthritis symptoms within 3 months of anti-TB treatment (OR, 33; 95% CI, 2.45~443.6) (
Table 2). In contrast, the presence of RF or anti-CCP and radiographic progression after 12 months was frequently observed in patients with RA flares.
Table 2
Comparisons between Poncet's disease and rheumatoid arthritis in active TB patients
|
Characteristics |
Poncet's disease
(n=12) |
Rheumatoid arthritis
(n=8) |
Odds ratio*
|
95% confidence interval |
|
Fever (≥38.3°C) at onset |
9 (75.0) |
1 (12.5) |
24.00 |
1.78~248.1 |
|
Symmetric involvement |
8 (66.7) |
4 (50.0) |
2.00 |
0.32~12.51 |
|
The presence of RF |
2 (16.7) |
6 (75.0) |
0.20 |
0.03~1.33 |
|
The presence of anti-CCP |
1 (8.3) |
5 (50.0) |
0.05 |
0.01~0.66 |
|
Response to NSAIDs alone |
10 (83.3) |
1 (12.5) |
35.00 |
2.63~465.4 |
|
Remission within 3 months after anti-TB treatment |
11 (100) |
2 (25.0) |
33.00 |
2.45~443.6 |
|
Radiographic progression after 12 months |
1 (8.3) |
4 (50.0) |
0.09 |
0.01~1.08 |

Crystal induced arthropathy was the most common inflammatory arthritis associated with active tuberculosis (
Table 1). Among the 14 patients with crystal-induced arthropathy, 11 (78.6%) had gout and 3 (21.4%) had pseudogout. Male patients (90.9%) or younger age groups (<65 years, 54.5%) were more common in gout cases than in pseudogout cases (both p<0.05). Similar to TB arthritis, crystal-induced arthropathy mainly presents as monoarthritis. However, small-to-medium-sized joints were predominantly involved in crystal-induced arthropathy when compared to TB arthritis (57.1% vs. 16.7%, p=0.013). Arthritis attacks developed after the diagnosis of TB infection, except for one patient with pseudogout, which coexisted with TB arthritis at the same joint (knee). Hyperuricemia (serum uric acid >7 mg/dL) was detected in 8 of 11 patients (median, 9.8 mg/dL; IQR, 7.4~14.8) who had experienced an attack of gouty arthritis before active TB infection, but only 1 patient had taken a uric acid-lowering agent (allopurinol 200 mg daily). In all gout cases, arthritis attacks occurred 4 weeks after the start of anti-TB treatment.
Go to :

DISCUSSION
TB infection and its associated conditions can cause uncommon but clinically significant problems in the musculoskeletal system [
2,
8,
12]. In the present study, 149 (5.2%) of 2,872 patients with active TB infection only complained of arthralgia, with one-third of them having inflammatory arthropathy. There were 12 patients with TB arthritis, accounting for 0.4% of all cases. TB arthritis is rare and often misdiagnosed as a degenerative condition or an inflammatory arthropathy such as RA due to its chronic and indolent disease course, as well as a lack of isolation of the organism in routine culture of the synovial fluid [
13,
14]. Moreover, it is difficult to distinguish TB arthritis from various forms of inflammatory arthropathy during active TB infection. We found that the majority of the inflammatory arthritis cases, except crystal-induced arthropathy, revealed joint symptoms prior to the diagnosis of active TB infection. In this regard, recognition of TB infection has become a challenge for physicians when patients with active TB infection have arthralgia as an initial or primary symptom.
The typical pattern of TB arthritis is monoarthritis involving weight-bearing joints such as the hip and knee [
2]. Although TB arthritis occurring in the upper extremities is rare [
15], one-third of the TB arthritis cases in the present study occurred in the elbow, wrist, or metacarpophalangeal joints. To confirm TB arthritis, synovial fluid aspiration or synovial biopsy is necessary for microbiological tests, such as AFB smear/culture and TB PCR or histopathologic examination [
16]. However, synovial fluid analysis, such as white blood cell counts, shows variable or nonspecific findings; it may present predominance of either neutrophils or lymphocytes [
17]. Moreover, the AFB smear of synovial fluid for TB arthritis has been reported to be positive in only 20% of cases, but it was much higher for cultures, with up to 80% of cases being positive [
14]. Molecular diagnostic assays using PCR have proven to be valuable for the rapid diagnosis of pulmonary TB, but their application in extrapulmonary TB remains limited. A previous study showed limited sensitivity (62.5%) and specificity (91.66%) in non-spinal samples, including various synovial tissues and synovial fluid [
18]. Histopathologic findings such as granuloma formation alone may be insufficient for the diagnosis of TB [
19]. It requires professional expertise and takes over two weeks to obtain the report. Nevertheless, the histological examination may allow for an early presumptive diagnosis until AFB culture results are confirmed, which takes 4~6 weeks to be reported but have a high yield to confirm the histologic finding. In this respect, patients with unexplained chronic monoarthritis, even in the upper extremities, should be considered for a synovial biopsy for histopathologic examination and AFB culture to rule out TB arthritis.
PD has been described as inflammatory polyarthritis in the presence of active visceral TB without bacterial involvement of the joints. The most commonly affected joints are the ankle and knee, but wrists and small joints in the hand can also be involved [
10,
20]. As the diagnosis is largely based on clinical findings and exclusion of other causes of arthritis in patients with a history of active TB, PD can often be misdiagnosed as RA. Sharma et al. [
10] suggested the diagnostic criteria for PD (Sharma and Pinto’s criteria): essentially including inflammatory, non-erosive, and non-deforming arthritis and excluding other causes of inflammatory arthritis. Their major criteria include a concurrent diagnosis of extra-articular tuberculosis and complete response to anti-TB treatment. In this study, we diagnosed PD in a patient who satisfied both the aforementioned essential and major criteria. PD was also diagnosed in a patient with underlying RA, when the joint symptoms improved with anti-TB treatment despite the disease-modifying antirheumatic drugs (DMARDs) being discontinued. In patients with RA flare, joint symptoms did not improve even after adequate anti-TB treatment, but were relieved after starting a new DMARD. Patients were excluded if there was insufficient evidence for diagnosis.
In our study, PD exhibited a higher proportion of polyarthritis and involved small-to-medium-sized joints as well as large joints (mixed distribution) when compared to TB arthritis. Patients with pre-existing RA developed oligo- or polyarthritis, but the disease was more likely to affect small-to-medium-sized joints, rather than a mixed distribution as seen in patients with PD. The duration of the resolution of arthritis symptoms after starting anti-TB treatment was significantly shorter in patients with PD than in those with TB arthritis. In addition, when compared to RA patients, there were more PD patients with arthritis remission within 3 months after anti-TB treatment and a good response to NSAIDs alone. The presence of anti-CCP may also be useful in distinguishing RA flares from PD. However, serological tests for RF or anti-CCP can be positive among active TB patients [
21,
22]. We diagnosed two patients with positive RF or anti-CCP as PD because arthralgia was spontaneously relieved with anti-TB treatment.
In all gouty cases, the arthritis attacks developed early after the diagnosis of TB infection and was associated with the initiation of anti-TB treatment. The incidence of gouty attacks on anti-TB treatment has been reported to be rare, and it is well known that gouty attacks have been associated with patients taking pyrazinamide or ethambutol, both of which induce hyperuricemia by decreasing renal uric acid clearance [
12,
23]. In our cohort study, the incidence of gouty attack accompanying active TB infection was only 0.4% of all cases, and approximately three-quarters of patients had a prior history of gout but did not regularly take uric acid-lowering agents. Furthermore, the majority of patients experienced the gouty attack within 2 months of initiating anti-TB treatment, which might be because anti-TB medications such as pyrazinamide or ethambutol affects the rapid increase or fluctuation of serum uric acid levels. A recent study revealed that higher body mass index, chronic kidney disease, and pre‐treatment hyperuricemia were independent risk factors for gouty attack while taking anti-TB medication [
12]. It is generally unnecessary to prevent or treat drug-induced hyperuricemia because it is often asymptomatic, and the change in serum uric acid levels is reversible after the withdrawal of pyrazinamide or ethambutol. However, our findings indicate that gouty patients with untreated hyperuricemia may be prone to an acute arthritis attack after initial intensive combination treatment against active TB infection. When initiating anti-TB treatment in patients with a previous gouty attack, physicians should consider proper management of pre-treatment hyperuricemia to prevent acute attack of gouty arthritis during anti-TB treatment.
We found several patients who experienced RA flare-ups accompanying active TB infection, one of whom was newly diagnosed with RA. Treatment of RA patients with biological agents can promote active TB infection or reactivate latent TB infection [
8,
9], but it is unclear whether TB infection aggravates autoimmune diseases such as RA. Based on the evidence of molecular mimicry between the pathogen and host antigens, previous studies have reported that autoimmunity can be triggered by TB infection [
24]. TB infection may also be associated with the emergence of various autoantibodies such as RF, anti-nuclear antibody, anti-neutrophil cytoplasmic antibodies, and anti-phospholipid antibodies [
25]. An epidemiological survey in Taiwan showed associations of TB infection with an increased prevalence of RA but was unable to establish a causal relationship [
26]. A previous study reported that in a collagen-induced mouse model, arthritis phenotypes are enhanced by the administration of killed
M. tuberculosis, which promotes interleukin-6 expression in macrophages via toll-like receptor 2 [
27]. Most published studies are based on epidemiological surveillance or experimental models. Further prospective cohort studies are needed to clarify whether active TB infection can exacerbate or promote RA.
The association of TB arthritis in patients with RA has been reported for several decades [
28,
29]. However, due to similar radiographic findings and clinical manifestations, it can be difficult to distinguish between RA and TB arthritis [
5]. We observed that fewer TB patients with RA flare-ups had complained of fever than TB patients with PD. One of the contributing factors may be the unintended antipyretic effect of anti-inflammatory agents such as NSAIDs and glucocorticoids (GCs), which improve the symptoms of active RA. Furthermore, GC use not only increases the risk of developing TB but also masks constitutional symptoms suggestive of active infection [
30,
31]. Active TB infection should be considered as the cause of fever of unknown origin, but the fever can sometimes be low-grade or absent, particularly in the elderly [
32]. Unlike septic arthritis from other etiologies, TB arthritis usually presents as a slowly progressive monoarthritis, which can be easily mistaken for disease flare-up in RA patients taking NSAIDs or GCs. The interferon-gamma release assay (IGRA), one of the screening tests for latent TB infection, may report false-negative or indeterminate results despite its high specificity. A meta-analysis reported that approximately 8%~19% of patients had a negative IGRA result when presenting with active TB [
33], and it revealed suboptimal sensitivity in patients with immunodeficiency, advanced age, and extrapulmonary TB [
34-
36]. In our cohort study, three RA patients who were diagnosed with active TB infection showed negative IGRA and no evidence of constitutional or respiratory symptoms. They were treated with a more intensive combination of DMARDs, including TNF-α inhibitors, to control arthritis symptoms, which resulted in rapid activation of TB. As culture-positive pulmonary TB with a normal chest X-ray (CXR) is not uncommon [
37], repeated sputum studies and serial follow-up CXRs can be helpful in the detection of active TB in RA patients with highly susceptible conditions when considering the biologics for arthritis control, even with negative IGRA results.
The main limitation of our study was its single-center retrospective design. We excluded 34 patients who had joint pain but had insufficient data for diagnosis, and only 47 patients with inflammatory arthritides accompanying active TB infection were identified and analyzed, resulting in low statistical power. Nevertheless, to our knowledge, this is the first study to analyze the prevalence of inflammatory arthritides in all active TB patients in a single center. In addition to our systematic analysis of the clinical features of TB-associated arthritis, we also compared it with RA. Further multicenter prospective observational studies are needed to determine the association between TB and RA.
Go to :





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download