Introduction
Aim of the work
Material and Methods
Drug
Experimental animals
The study design
-
Group I (adult control): six rats were further divided into two subgroups:
Group II (adult ciprofloxacin treated rats): six rats were treated with ciprofloxacin in a dose of 3.5 mg/kg/d for 14 days [17].
-
Group III (senile control): six rats were further divided into two subgroups:
Group IV (senile ciprofloxacin treated rats): six rats were treated with ciprofloxacin as in group II.
Collection of samples for light microscopic examination
Immunohistochemical study
Preparation of samples for scanning electron microscopic examination
Statistics and image analysis
Ethical consideration
Results
Histological and immunohistochemical results
Adult groups (groups I and II)
Hematoxylin and eosin stained sections
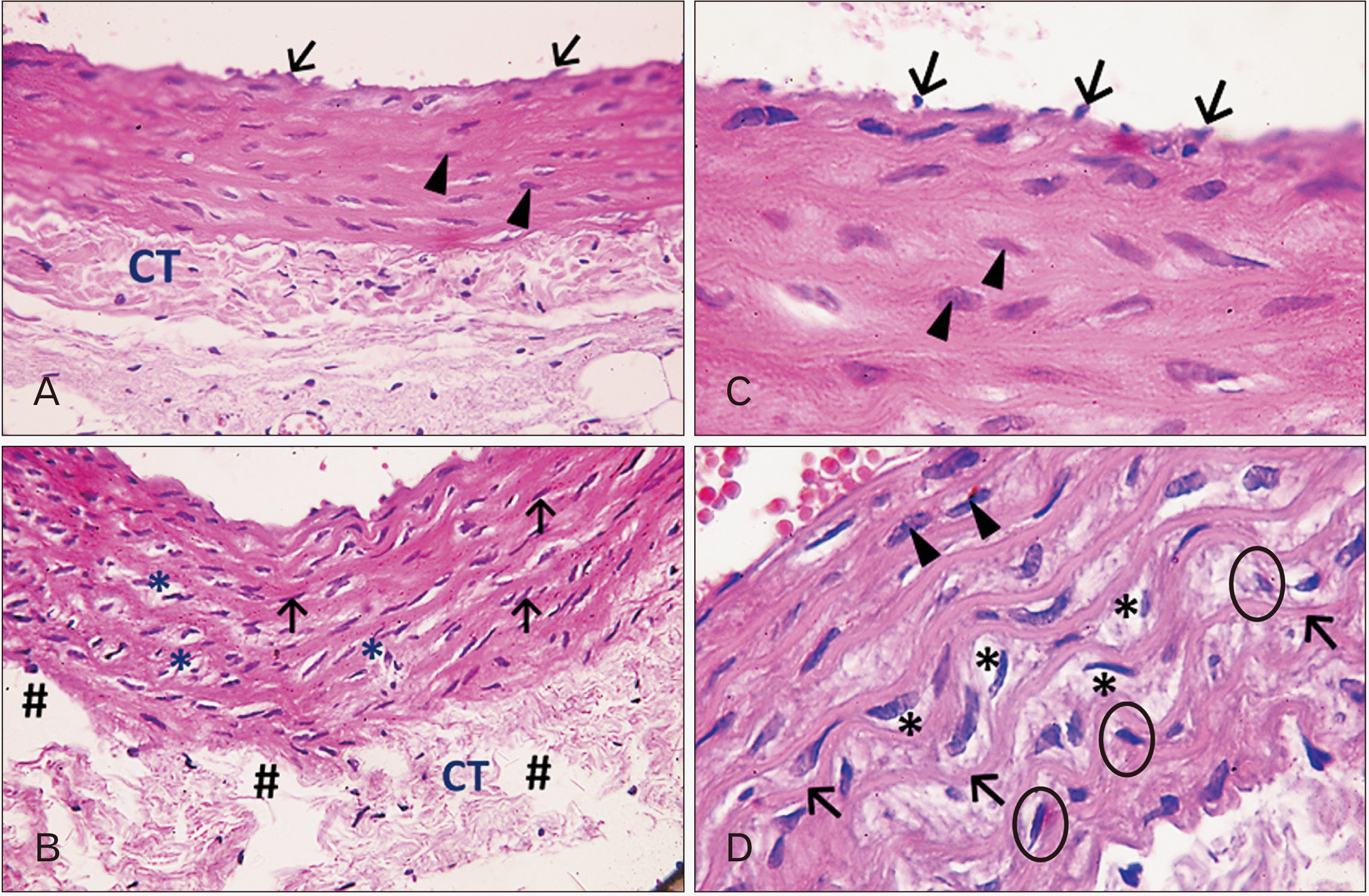 | Fig. 1Photomicrographs of sections in the thoracic aortae of adult rats (H&E). (A) Group I (adult control; ×400): the tunica intima shows simple squamous endothelial cells having flattened nuclei bulging into the lumen (arrows). The tunica media shows smooth muscle cells (SMCs) having single oval nuclei (arrowheads). Notice the packed, wavy connective tissue (CT) in the tunica adventitia. (B) Group II (adult rats treated with ciprofloxacin; ×400): tunica intima reveals uneven surface with many folds. Tunica media shows many vacuoles of lost tissue (asterisks), many nuclei of SMCs are darkly stained (pyknotic; arrows). The adventitia shows loosely packed CT with multiple tissue loss (hashtags). (C) Group I (adult control; ×1,000): the simple squamous cells of the tunica intima appear having flattened nuclei bulging into the lumen (arrows). The tunica media shows the oval nuclei (arrowheads) of SMCs that have regular concentric arrangement alternating with elastic laminae. (D) Group II (adult rats treated with ciprofloxacin; ×1,000): the inner part of the tunica media shows the classical concentric arrangement of the SMCs (arrowheads). The outer part of the tunica media exhibits attenuation of the SMCs (circles) with multiple areas of tissue loss (asterisks ) and folding of the elastic laminae (arrows). |
Orcein stained sections
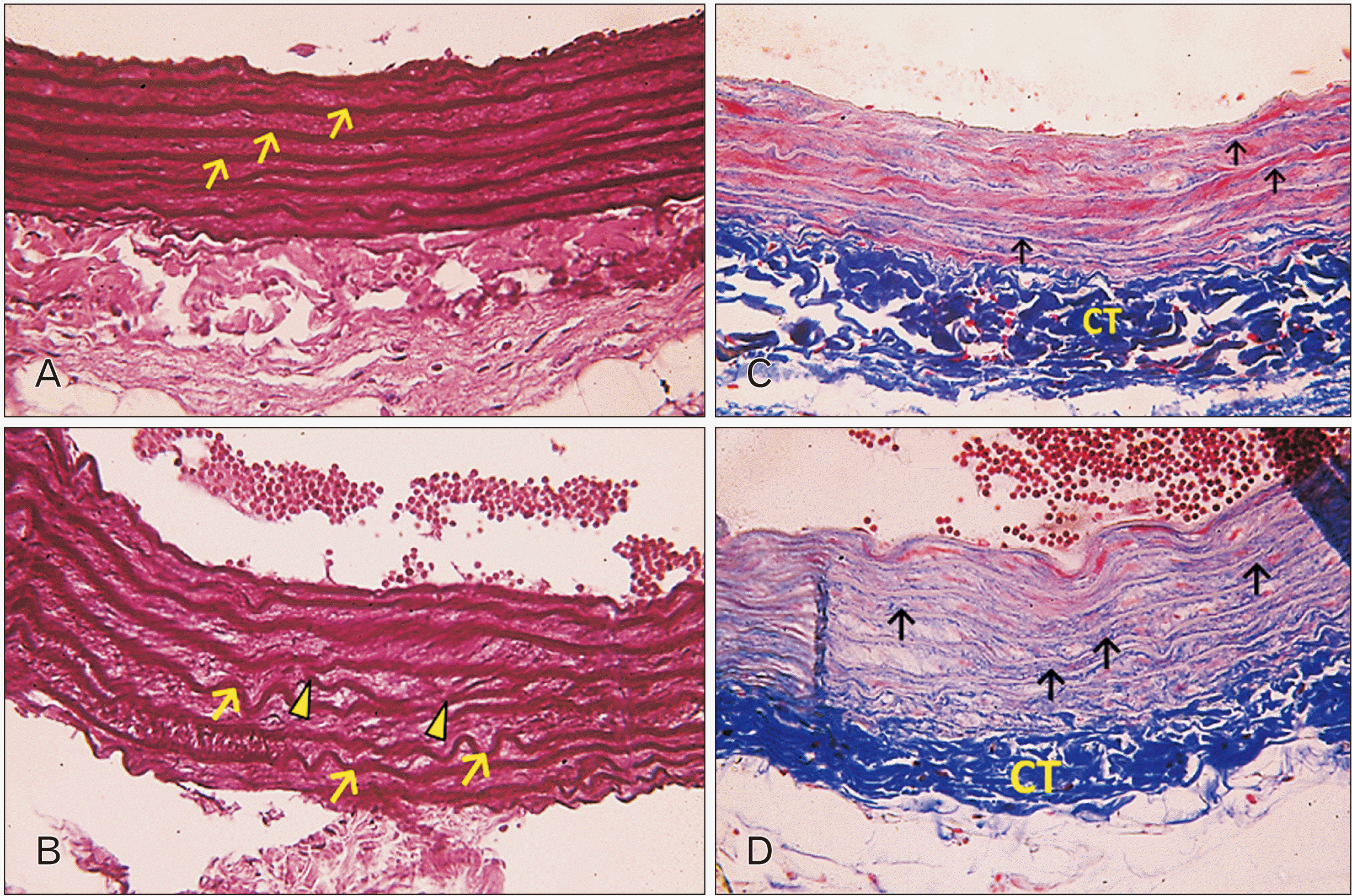 | Fig. 2Photomicrographs of sections in the thoracic aortae of adult rats. (A) Group I (adult control; Orcein, ×400): the laminae of elastic fibers are thick, parallel, and organized (arrows). (B) Group II (adult rats treated with ciprofloxacin; Orcein, ×400): the laminae of elastic fibers are corrugated (arrows) and fragmented (arrowheads). Also note the widening between the elastic laminae with partial loss of their regular arrangement. (C) Group I (adult control; Masson’s Trichrome, ×400): wavy collagen fibers (arrows) are seen with the smooth muscle cells (SMCs) distributed in between. Notice the packed connective tissue (CT) in tunica adventitia. (D) Group II (adult rats treated with ciprofloxacin; Masson’s Trichrome, ×400): note the aberrant increase in the collagen fibers deposition (arrows) that is encroaching on the SMCs in the tunica media. The SMCs are few, interrupted and show very faint acidophilic stain. Tunica adventitia shows very dense deposition of CT. |
Masson’s trichrome stained sections
Immunohistochemical staining for alpha smooth muscle actin
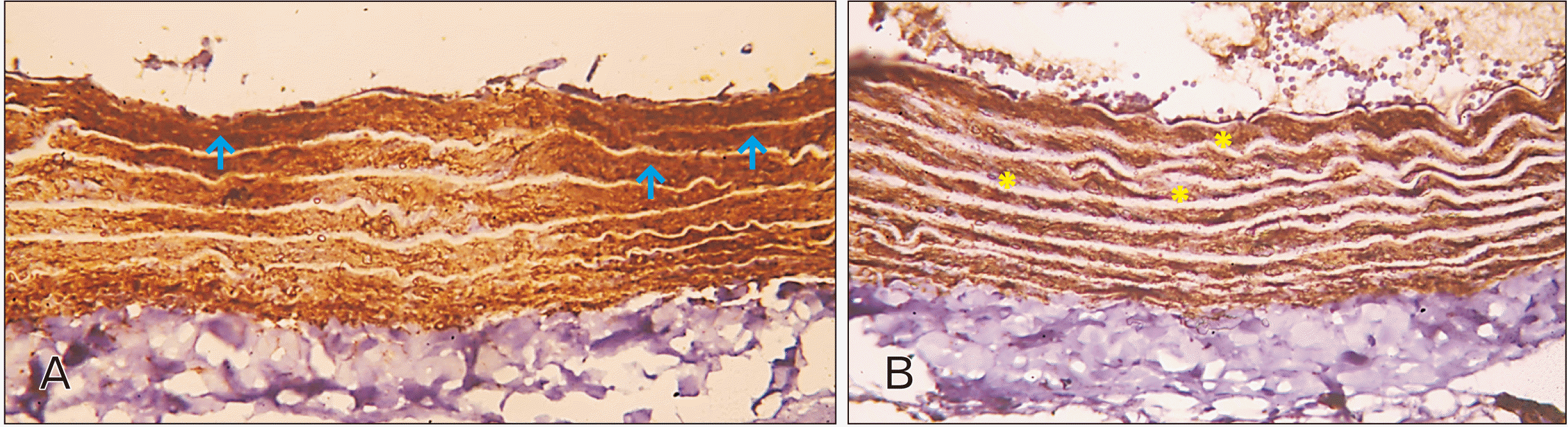 | Fig. 3Photomicrographs of sections in the thoracic aortae of adult rats (immune staining with anti-alpha smooth muscle actin antibody, ×400). (A) Group I (adult control): notice homogenous packed layers of strong positive alpha actin filaments of smooth muscle fibers (arrows) in tunica media located between elastic laminae. (B) Group II (adult rats treated with ciprofloxacin): weak positive immune reaction for alpha smooth muscle actin is observed (asterisks). |
Scanning electron microscope results
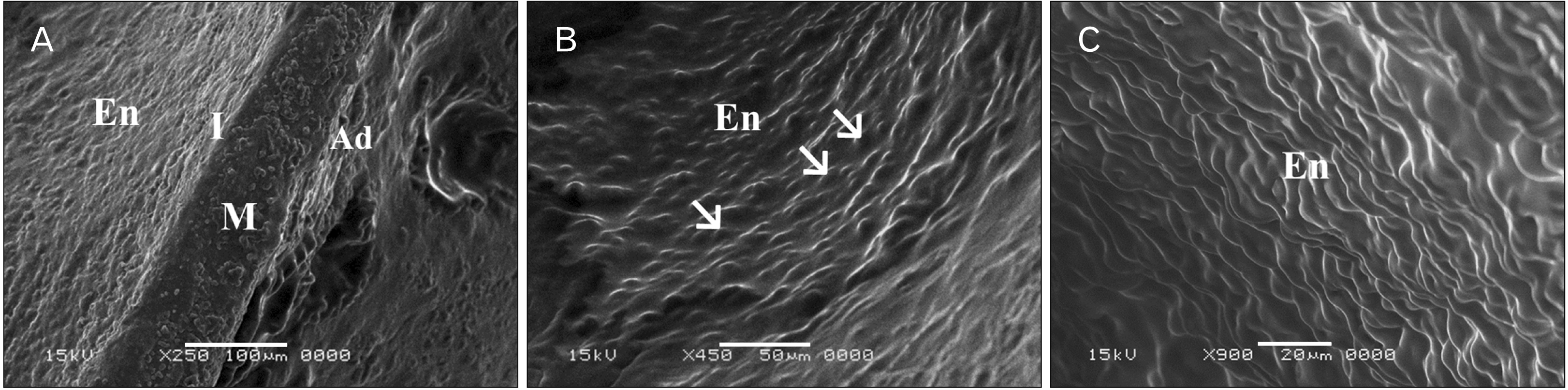 | Fig. 4Scanning electron microscope (SEM) photographs of sections in the thoracic aortae of adult rats. (A) Group I (adult control; ×250; scale bar, 100 μm): the vessel wall is formed of well adherent three layers; intima (I), media (M), and adventitia (Ad). The media is the thickest layer. Endothelial surface (En) appears smooth and homogenous. (B) Group I (adult control; ×450; scale bar, 50 μm): luminal En appears smooth and continuous showing nuclei (arrows) of the lining endothelium protruding into the vessel lumen with distinct boundaries between adjacent cells. The cells are aligned parallel to the long axis of the vessel. (C) Group II (adult rats treated with ciprofloxacin; ×900; scale bar, 20 μm): rough En with numerous irregular folds between the endothelial cells are seen. |
Senile groups (groups III and IV)
Hematoxylin and eosin stained sections
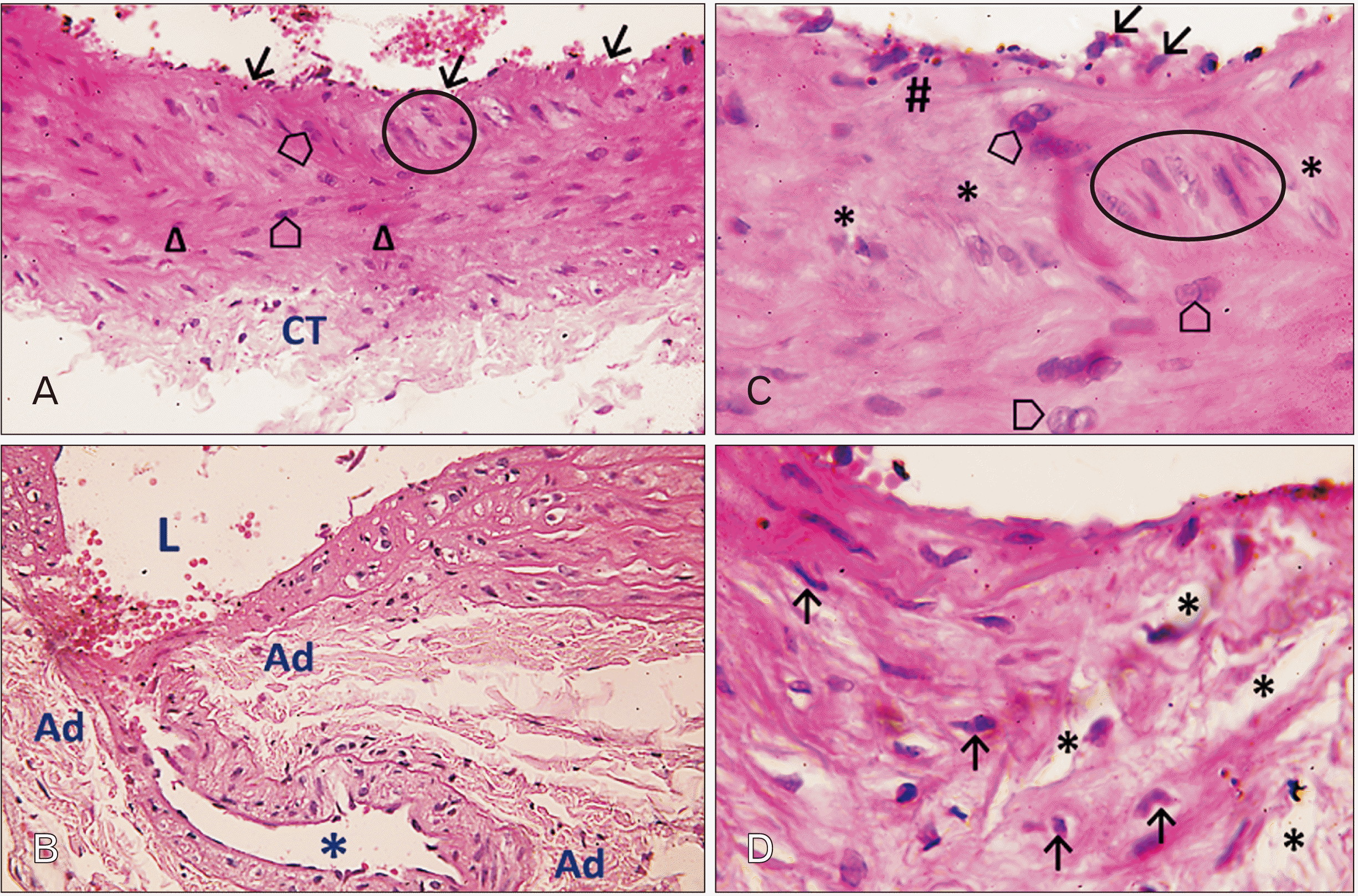 | Fig. 5Photomicrographs of sections in the thoracic aortae of senile rats (H&E). (A) Group III (senile control; ×400): a photomicrograph showing tunica intima with areas of desquamation (arrows). Notice the area of intimal thickening with vertically aligned nuclei of smooth muscle cells (SMCs; circle). The tunica media reveals lack of concentric arrangement of SMCs with spindle shaped nuclei (triangles). Binucleated proliferating SMCs can be seen (pentagons). Notice also the sparse, loose connective tissue (CT) in the tunica adventitia. (B) Group IV (senile rats treated with ciprofloxacin; ×400): notice the outpouching of the aortic wall made of an invagination of the inner layers of aortic wall outwards (asterisk). The pouch is connected to aortic lumen by a narrow area of intimal tear. The tunica media is severely damaged with numerous pyknotic nuclei and vacuolated SMCs. The tunica media thins out at the outpouching. Ad, adventitia; L, lumen of the vessel. (C) Group III (senile control; ×1,000): tunica intima reveals un-even endothelial lining with some detached lining cells (arrows). Discontinuous internal elastic lamina is seen (hashtag). An area of intimal thickening with vertically aligned nuclei of SMCs can also be seen (circle). The tunica media shows random arrangement of SMCs nuclei, pale staining degenerating myocytes, and frequent patches of muscle tissue loss (asterisks). Proliferating binucleated SMCs are seen (pentagons). (D) Group IV (senile rats treated with ciprofloxacin; ×1,000): notice the tunica media with severe damage of SMCs with pyknotic nuclei (arrows). Notice the disarrangement of smooth muscle fibers and spacing due to tissue loss (asterisks). |
Orcein stained sections
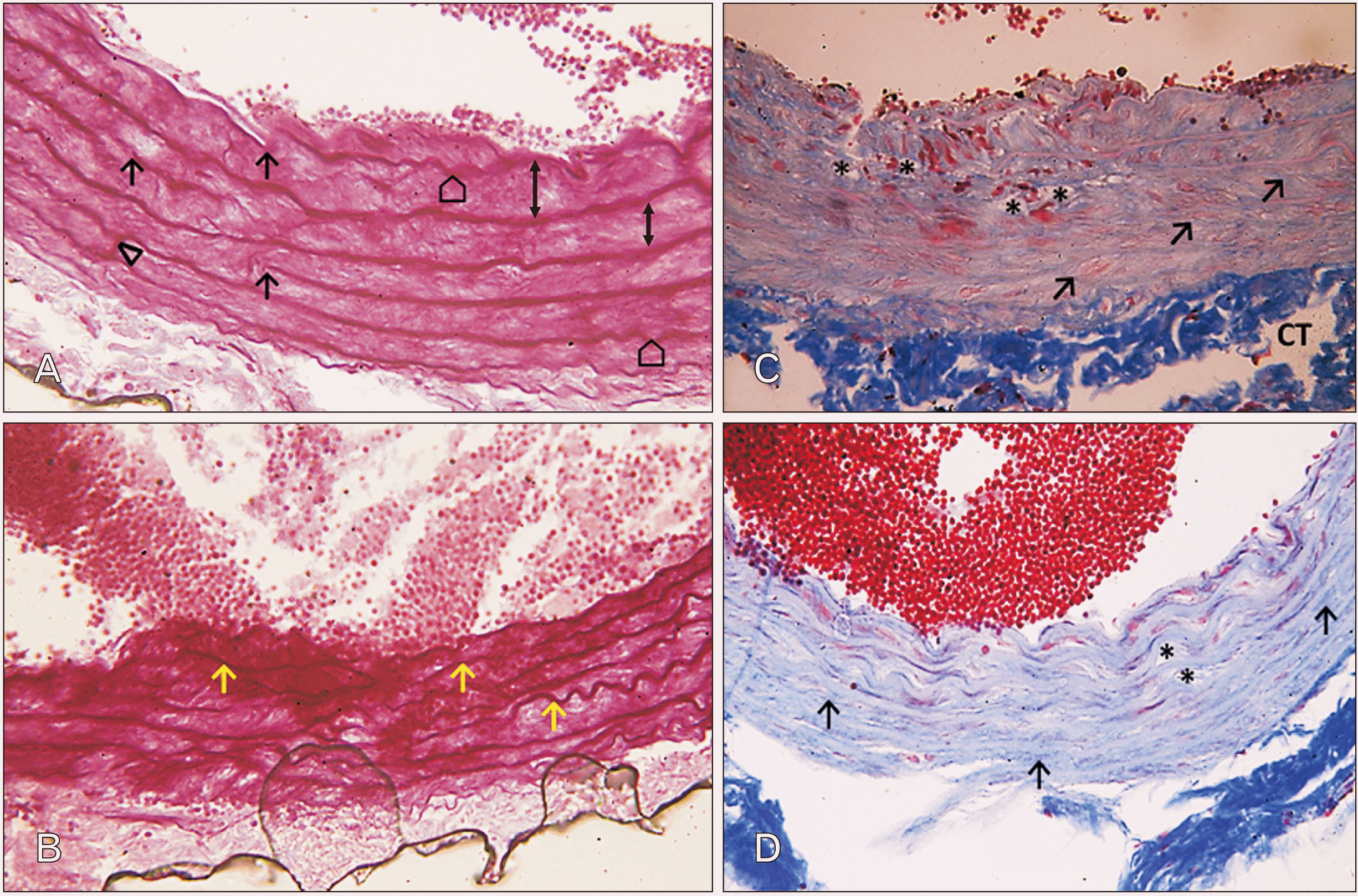 | Fig. 6Photomicrographs of sections in the thoracic aortae of senile rats. (A) Group III (senile control; Orcein, ×400): the elastic fibers appear widely spaced (up down arrows), fragmented (arrows) and corrugated (pentagons). Branching of elastic laminae (triangle) can also be visualized. (B) Group IV (senile rats treated with ciprofloxacin; Orcein, ×400): notice the marked disruption of the regular arrangement of the elastic laminae. Some of them appear severely corrugated or fragmented (arrows). (C) Group III (senile control; Masson’s Trichrome, ×400): many of collagen fibers (arrows) are seen with sparse acidophilic smooth muscle cells in tunica media. Vacuoles (asterisks) could be seen occupying interlamellar spaces. Notice the widely separated connective tissue (CT) in tunica adventitia. (D) Group IV (senile rats treated with ciprofloxacin; Masson’s Trichrome, ×400): notice the aberrant increase in the collagen fibers’ deposition (arrows) encroaching on the muscle layer in tunica media. Vacuoles (asterisks) could be also seen occupying interlamellar spaces. Notice the aberrant decrease in the vessel wall thickness. |
Masson’s trichrome stained sections
Immunohistochemical staining for alpha smooth muscle actin
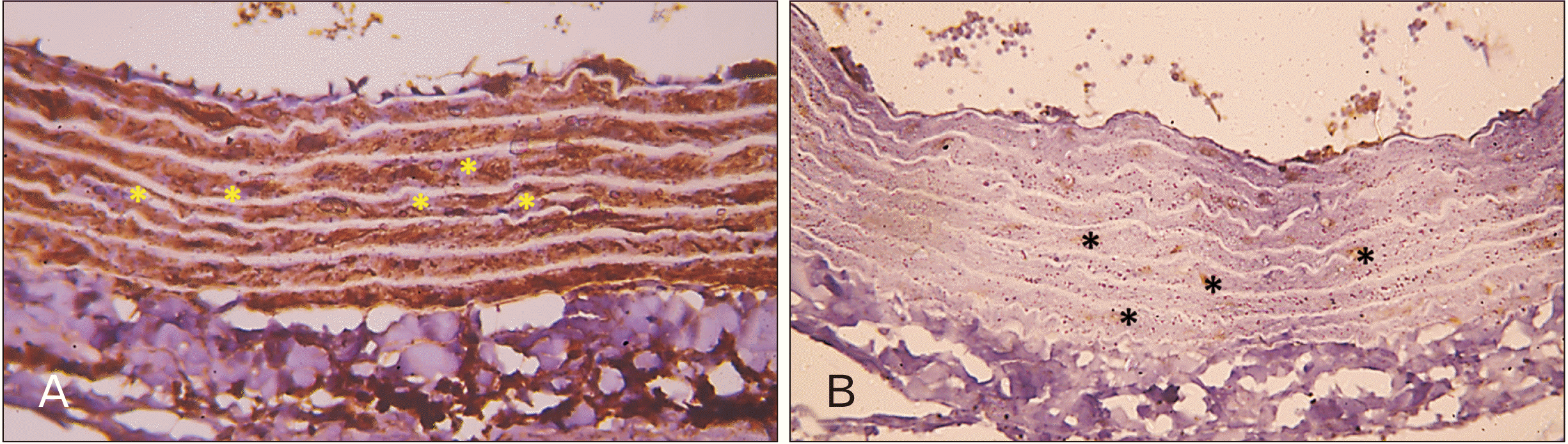 | Fig. 7Photomicrographs of sections in the thoracic aortae of senile rats (immune staining with anti-alpha smooth muscle actin antibody, ×400). (A) Group III (senile control): moderate to weak immune reactivity (asterisks) is seen in many patches through the tunica media. (B) Group IV (senile rats treated with ciprofloxacin): almost negative immune reactivity of smooth muscle fibers is seen through the whole extent of the tunica media with only sporadic patches of moderate immune reactivity appearing as brown color (asterisks). |
Scanning electron microscope results
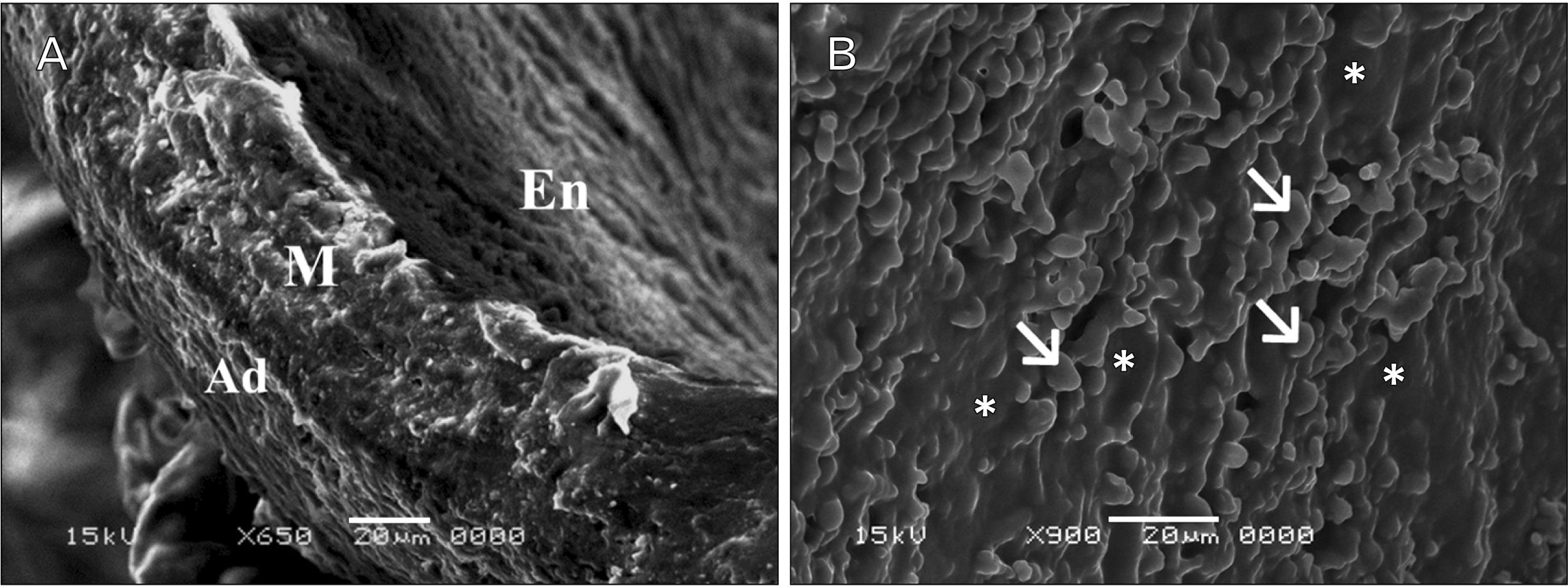 | Fig. 8Scanning electron microscope (SEM) photographs of sections in the thoracic aortae of senile control rats (group III; scale bar: 20 μm). (A) Notice the rough endothelial lining (En) (×650). The media (M) is the thickest layer with outer adventitial layer (Ad). The three layers are tightly opposed to each other. (B) Luminal endothelial surface appears rough with irregular folds between endothelial cells (arrows) (×900). Notice the thinning of endothelium (asterisks) in some areas. |
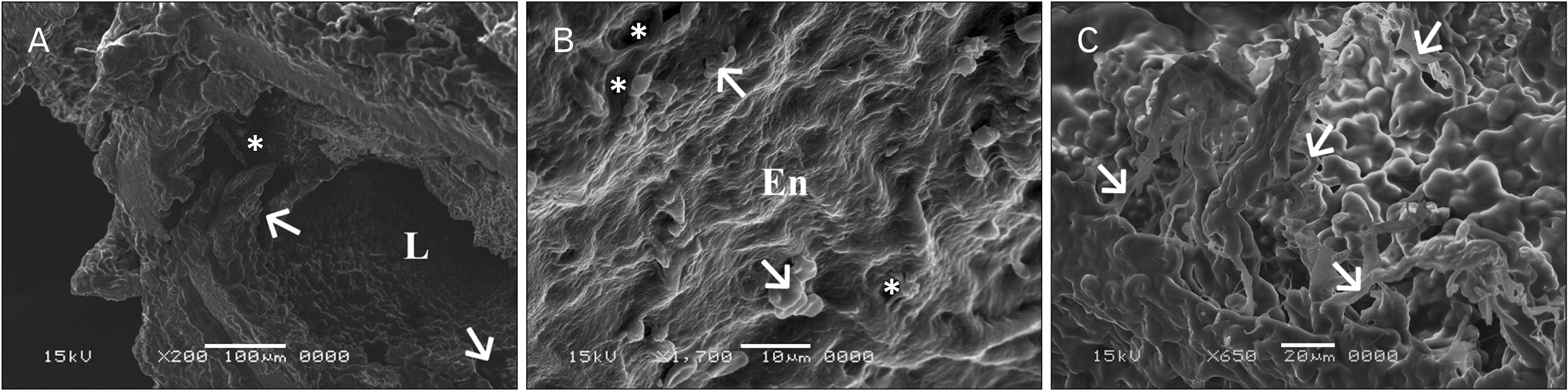 | Fig. 9Scanning electron microscope (SEM) photographs of sections in the thoracic aortae of senile rats treated with ciprofloxacin (group IV). (A) Notice outpouching (asterisk) of the vessel wall extending towards the tunica adventitia (×200; scale bar, 100 μm). The intima reveals many cracks (arrows). L, vessel lumen. (B) Notice the rough endothelial lining (En) with the presence of many fissures or cracks (asterisks) (×1,700; scale bar, 10 μm). Some areas show adherent blood cells (arrows). (C) Notice the accumulation of fibrin (arrows) (×650; scale bar, 20 μm). |
Morphometric results and statistics
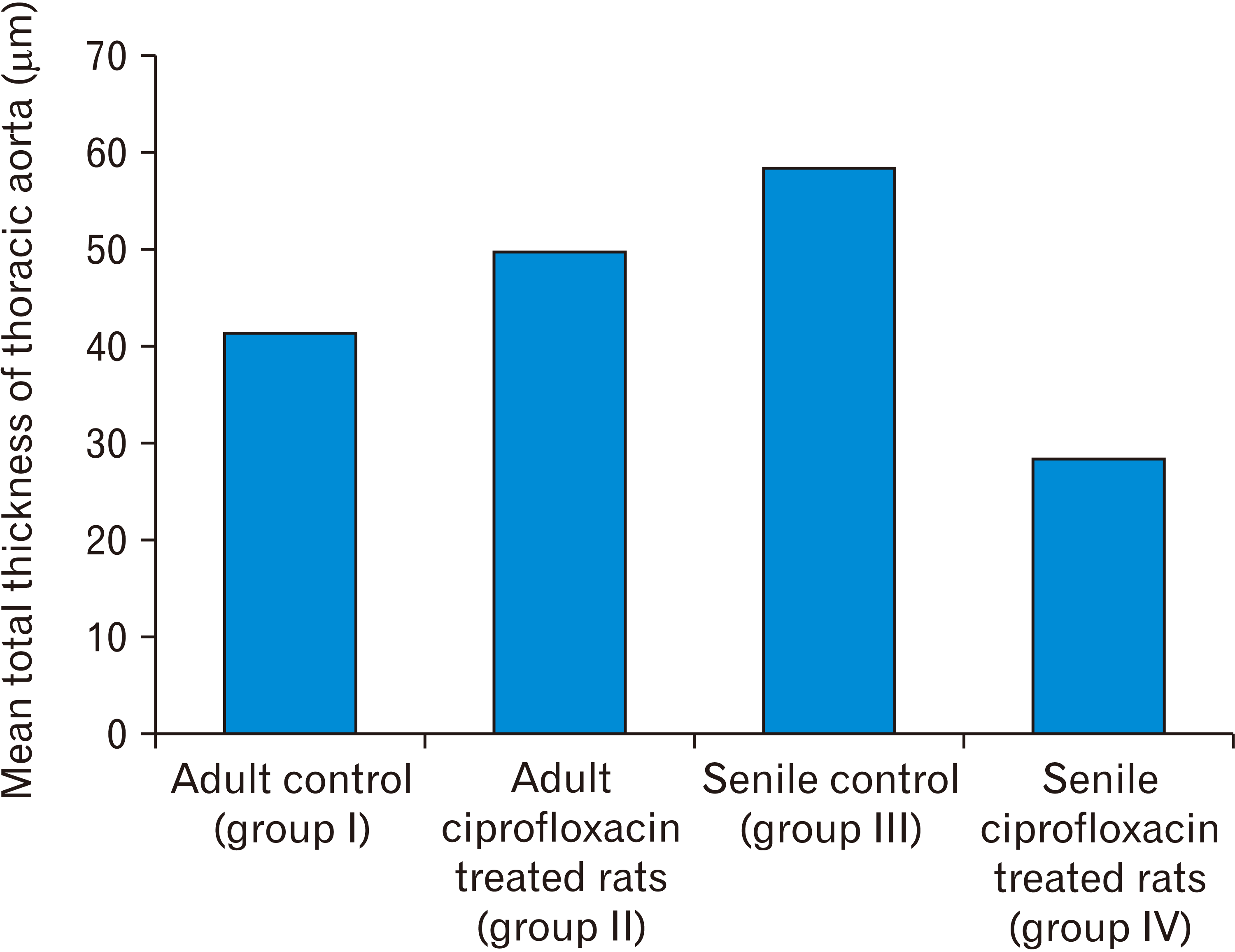
The total thickness of the wall of the thoracic aorta
Table 1
| Total thickness of the wall of thoracic aorta (μm) | Adult control (group I) | Adult ciprofloxacin treated rats (group II) | Senile control (group III) | Senile ciprofloxacin treated rats (group IV) |
|---|---|---|---|---|
| Mean±standard deviation | 44.08±4.7 |
52.53±3.12 (P=0.0004)a) |
61.1±2.46 (P<0.001)a) (P<0.001)b) |
31.15±6.07 (P=0.00012)c) (P<0.001)d) (P<0.001)e) |
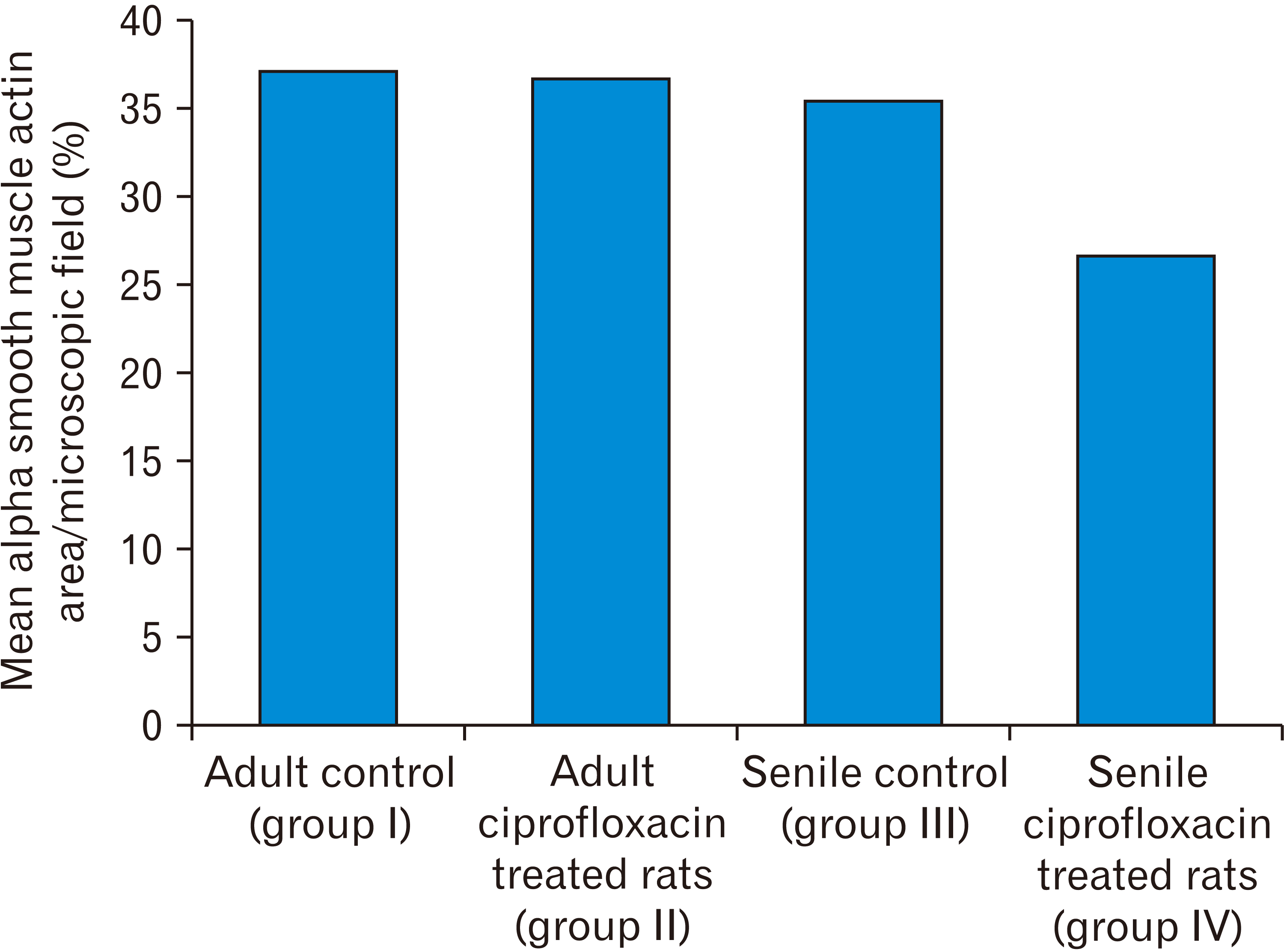
Mean area percentage of alpha smooth muscle actin staining per microscopic field
Table 2
| Alpha smooth muscle actin staining area per microscopic field (%) | Adult control (group I) | Adult ciprofloxacin treated rats (group II) | Senile control (group III) | Senile ciprofloxacin treated rats (group IV) |
|---|---|---|---|---|
| Mean±standard deviation | 38.78±0.9 | 38.34±1.9 |
37.09±0.46 (P=0.006)a) |
28.31±3.79 (P=0.00014)b) (P=0.00035)c) (P<0.00043)d) |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download