Introduction
Materials and Methods
Search strategy
Inclusion and exclusion criteria
Data extraction
Statistical analysis
Results
Characteristics of included studies
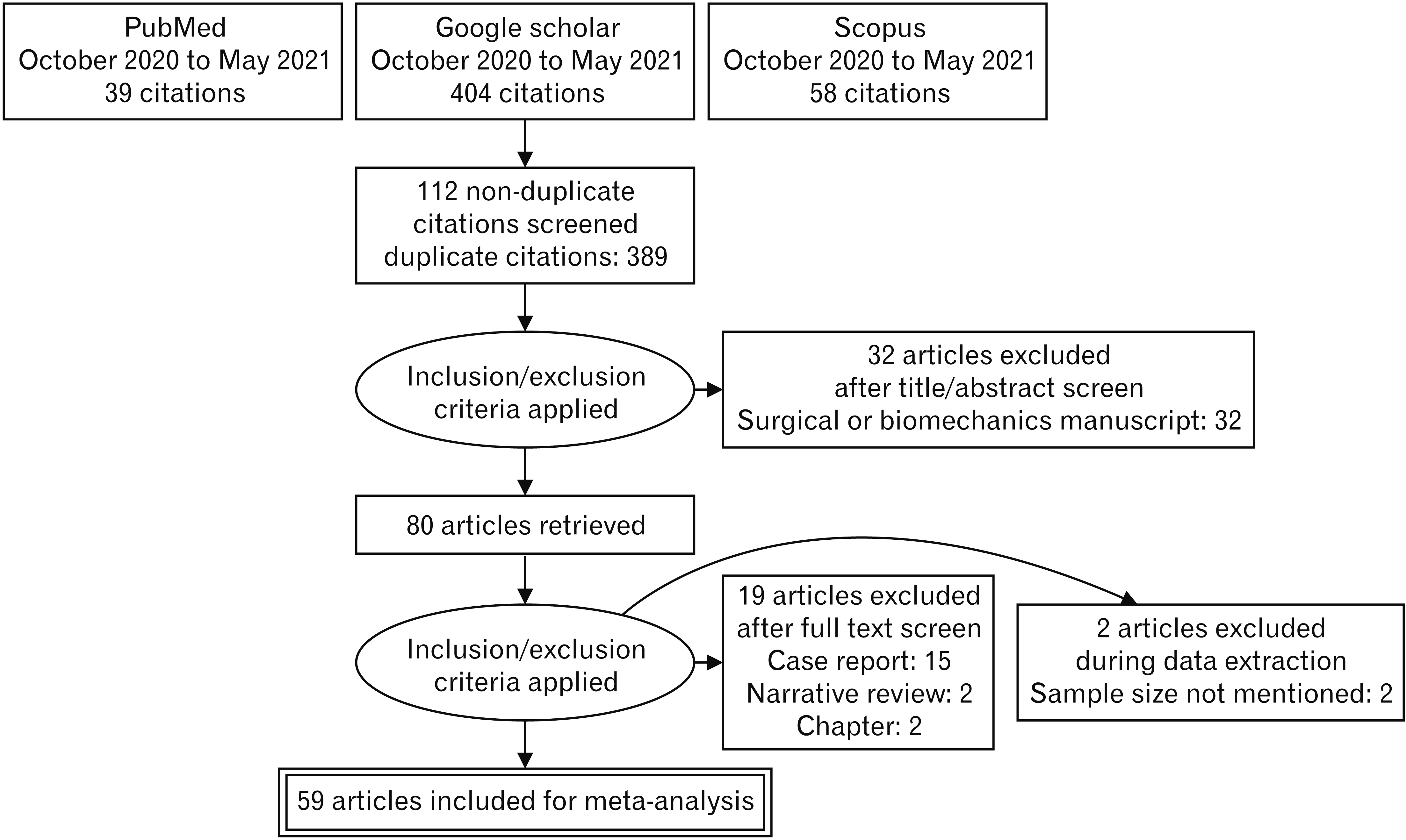 | Fig. 1Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram of search strategy for Gantzer’s muscle. |
Table 1
| Reference | Year | Prevalence (%) | 95% confidence interval | ahFPL/ahFDP | No. and ethnicity of sample | Risk of bias |
|---|---|---|---|---|---|---|
| Adachi [53]a) | 1910 | 63 | 54–70 | 84 | 134 Asian Mongoloid | Unclear |
| Afroze et al. [18] | 2020 | 24 | 14–38 | 12 | 50 Asian Caucasian | High |
| al-Qattan [15] | 1996 | 52 | 33–70 | 13 | 25 Asian Caucasian | Low |
| Bagoji et al. [16] | 2017 | 29 | 19–42 | 17 | 58 Asian Caucasian | Moderate |
| Bajpe et al. [17] | 2015 | 24 | 14–38 | 12 | 50 Asian Caucasian | High |
| Ballesteros et al. [19] | 2019 | 32 | 24–42 | 34 | 106 South American | Low |
| Bando [53]a) | 1956 | 64 | 59–69 | 217 | 340 Asian Mongoloid | Unclear |
| Bangarayya et al. [20] | 2018 | 40 | 24–58 | 12 | 30 Asian Caucasian | Moderate |
| Bilecenoglu et al. [21] | 2005 | 20 | 9–38 | 6 | 30 Asian Caucasian | Low |
| Burute and Vatsalaswamy [22] | 2017 | 36 | 29–44 | 56 | 156 Asian Caucasian | High |
| Caetano et al. [23] | 2015 | 68 | 57–77 | 54 | 80 South American | Low |
| Chakravarthi et al. [24] | 2014 | 72 | 59–83 | 39 | 54 Asian Caucasian | Moderate |
| Dubois de Monto-Marin et al. [55] | 2021 | 11 | 4–26 | 4 | 36 European Caucasian | Moderate |
| Dellon and Mackinnon [25] | 1987 | 33 | 20–48 | 14 | 43 North American | Low |
| Desai et al. [26] | 2017 | 58 | 46–70 | 35 | 60 Asian Caucasian | High |
| Dolderer et al. [27] | 2011 | 26 | 11–50 | 5 | 19 European Caucasian | Low |
| Dykes and Anson [28] | 1944 | 53 | 45–61 | 80 | 150 North American | Moderate |
| El Domiaty et al. [8] | 2008 | 62 | 47–75 | 26 | 42 African | Low |
| Gunnal et al. [29] | 2013 | 51 | 44–58 | 92 | 180 Asian Caucasian | Moderate |
| Hemmady et al. [30] | 1993 | 67 | 53–78 | 36 | 54 Asian Caucasian | Low |
| Herrold et al. [31] | 2020 | 55 | 49–60 | 148 | 271 South American | High |
| Inoue [53]a) | 1934 | 71 | 61–79 | 71 | 100 Asian Mongoloid | Unclear |
| Jones et al. [3] | 1997 | 45 | 34–56 | 36 | 80 European Caucasian | Low |
| Kara et al. [32] (adult) | 2012 | 38 | 26–52 | 20 | 52 Asian Caucasian | Low |
| Kara et al. [32] (fetal) | 2012 | 32 | 23–43 | 29 | 90 Asian Caucasian | Low |
| Khade et al. [33] | 2020 | 53 | 36–70 | 16 | 30 Asian Caucasian | Moderate |
| Kida [34] | 1988 | 62 | 54–70 | 82 | 132 Asian Mongoloid | Low |
| Kudo and Obata [53]a) | 1957 | 55 | 48–61 | 118 | 216 Asian Mongoloid | Low |
| Kumari et al. [35] | 2017 | 42 | 29–56 | 20 | 48 Asian Caucasian | Moderate |
| Le Double and Berry [36] | 1897 | 33 | 28–39 | 100 | 300 European Caucasian | Moderate |
| Loth [53]a) | 1912 | 89 | 78–95 | 50 | 56 African | Low |
| Mahakkanukrauh et al. [37] | 2004 | 62 | 56–68 | 149 | 240 Asian Mongoloid | Moderate |
| Malhotra et al. [38] | 1982 | 54 | 48–60 | 130 | 240 North American | Moderate |
| Mangini [10] | 1960 | 74 | 63–82 | 56 | 76 North American | Low |
| Matsunaga et al. [39] | 2000 | 35 | 27–43 | 50 | 144 Asian Mongoloid | Low |
| Mohammed [9] | 2018 | 64 | 52–76 | 38 | 59 African | Low |
| Mori [41] | 1964 | 50 | 43–57 | 103 | 205 Asian Mongoloid | Low |
| Mustafa et al. [40] | 2016 | 45 | 25–66 | 9 | 20 Asian Caucasian | Moderate |
| Oh et al. [51] | 2000 | 67 | 55–77 | 48 | 72 Asian Mongoloid | Moderate |
| Oliveira et al. [11] | 2021 | 50 | 34–66 | 17 | 34 South American | Low |
| Pai et al. [12] | 2008 | 46 | 38–55 | 58 | 126 Asian Caucasian | Low |
| Philip and Dakshayani [13] | 2018 | 22 | 13–36 | 11 | 50 Asian Caucasian | Moderate |
| Ravi Prasanna et al. [42] | 2019 | 36 | 24–50 | 18 | 50 Asian Caucasian | High |
| Riveros et al. [43] | 2015 | 10 | 3–27 | 3 | 30 South American | Moderate |
| Sano [53]a) | 1931 | 70 | 38–90 | 7 | 10 Asian Mongoloid | Unclear |
| Sato [44] | 1969 | 25 | 22–29 | 151 | 604 Asian Mongoloid | Moderate |
| Sekizawa [53]a) | 1960 | 54 | 43–64 | 45 | 84 Asian Mongoloid | Unclear |
| Sharma et al. [45] | 2008 | 40 | 28–53 | 24 | 60 Asian Caucasian | Moderate |
| Shayo et al. [46] | 2015 | 42 | 30–54 | 26 | 62 Asian Caucasian | Low |
| Shirali et al. [47] | 1998 | 55 | 42–67 | 33 | 60 North American | Moderate |
| Tamang et al. [48] | 2013 | 25 | 16–37 | 15 | 60 Asian Caucasian | High |
| Tomizawa [53] | 1986 | 54 | 35–73 | 13 | 24 Asian Mongoloid | Moderate |
| Tubbs et al. [49] | 2006 | 20 | 8–43 | 4 | 20 North American | Low |
| Uyaroglu et al. [50] | 2006 | 52 | 39–65 | 27 | 52 Asian Caucasian | Moderate |
| Wagenseil [54] | 1936 | 73 | 65–79 | 103 | 142 Asian Mongoloid | Moderate |
| Wagenseil [54] | 1936 | 55 | 47–62 | 82 | 150 European Caucasian | Moderate |
| Wood [14] | 1868 | 61 | 49–72 | 44 | 72 European Caucasian | Low |
| Yang et al. [4] | 2017 | 48 | 37–59 | 35 | 73 Asian Mongoloid | Moderate |
| Yu et al. [52] | 2018 | 58 | 31–82 | 7 | 12 Asian Mongoloid | Moderate |
| Pooled weighted prevalence | 48 | 44–52 | 2,844 | 5,903 random effect model | ||
| Bando [53]a) | 1956 | 25 | 21–30 | 86b) | 340 Asian Mongoloid | Moderate |
| El Domiaty et al. [8] | 2008 | 14 | 7–28 | 6b) | 42 African | Unclear |
| Inoue [53]a) | 1934 | 29 | 21–39 | 29b) | 100 Asian Mongoloid | Unclear |
| Jones et al. [3] | 1997 | 18 | 11–27 | 14b) | 80 European Caucasian | Low |
| Kudo and Obata [53]a) | 1957 | 20 | 16–26 | 44b) | 216 Asian Mongoloid | Low |
| Mohammed [9] | 2018 | 5 | 2–15 | 3b) | 59 African | Low |
| Mangini [10] | 1960 | 3 | 1–10 | 2b) | 76 North American | Low |
| Oliveira et al. [11] | 2021 | 3 | 0–18 | 1b) | 34 South American | low |
| Pai et al. [12] | 2008 | 14 | 9–22 | 18b) | 126 Asian Caucasians | Low |
| Philip and Dakshayani [13] | 2018 | 22 | 13–36 | 11b) | 50 Asian Caucasians | Moderate |
| Sano [53]a) | 1930 | 23 | 14–36 | 13b) | 56 Asian Mongoloid | Unclear |
| Sekizawa [53]a) | 1960 | 21 | 14–31 | 18b) | 84 Asian Mongoloid | Unclear |
| Wagenseil [54] | 1936 | 26 | 20–34 | 37b) | 142 Asian Mongoloid | Moderate |
| Wagenseil [54] | 1936 | 10 | 6–16 | 15b) | 150 European Caucasian | Moderate |
| Wood [14] | 1868 | 7 | 3–16 | 5b) | 72 European Caucasian | Low |
| Pooled weighted prevalence | 17 | 13–21 | 302b) | 1,627 random effect model |
Prevalence
 | Fig. 2Pooled weighted prevalence of accessory head of flexor pollicis longus variant. ES, effect size (log-odds ratio); CI, confidence interval; W, weight of study (inverse variance); N, sample size. a)Secondary reference was used because the data collected from secondary reference due to inaccessibility of original manuscript. |
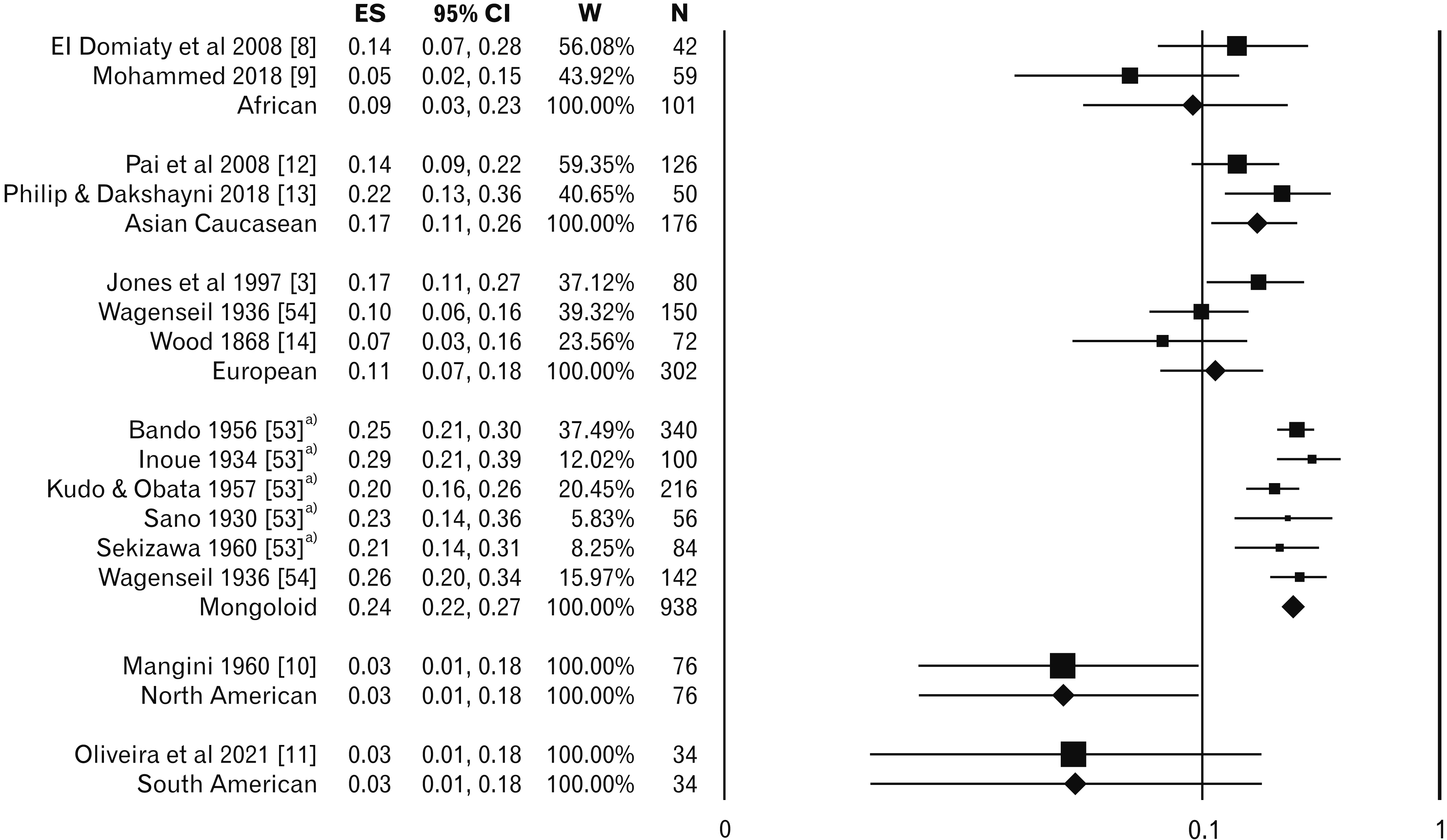 | Fig. 3Pooled weighted prevalence of accessory head of flexor digitorum profundus variant. ES, effect size (log-odds ratio); CI, confidence interval; W, weight of study (inverse variance); N, sample size. a)Secondary reference was used because the data collected from secondary reference due to inaccessibility of original manuscript. |
Ethnic and geographical distribution
Laterality and sex distribution
Table 2
Anatomical distribution
Table 3
Morphological distribution
Risk of bias
Publication bias
Discussion
Summary of findings
Agreement or disagreement with other studies
Type I: ahFPL
Type Ia: Insertion into the belly of FPL
Type Ib: Insertion into the tendon of FPL
Type II: ahFDP
Type IIa: Insertion into the first tendon of FDP (index finger)
Type IIb: Insertion into the second tendon of FDP (middle finger)
Type IIc: Insertion into the third tendon of FDP (ring finger)
Type IId: Insertion into the fourth tendon of FDP (little finger)




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download