2. Chen L, Qu J, Xiang C. 2019; The multi-functional roles of menstrual blood-derived stem cells in regenerative medicine. Stem Cell Res Ther. 10:1. DOI:
10.1186/s13287-018-1105-9. PMID:
30606242. PMCID:
PMC6318883.

3. Bellelis P, Podgaec S, Abrão MS. 2011; Environmental factors and endometriosis. Rev Assoc Med Bras (1992). 57:448–52. DOI:
10.1016/S0104-4230(11)70093-8.

4. Persoons E, De Clercq K, Van den Eynde C, Pinto SJPC, Luyten K, Van Bree R, Tomassetti C, Voets T, Vriens J. 2020; Mimicking Sampson's retrograde menstrual theory in rats: a new rat model for ongoing endometriosis-associated pain. Int J Mol Sci. 21:2326. DOI:
10.3390/ijms21072326. PMID:
32230898. PMCID:
PMC7177935.

5. Lv H, Hu Y, Cui Z, Jia H. 2018; Human menstrual blood: a renewable and sustainable source of stem cells for regenerative medicine. Stem Cell Res Ther. 9:325. DOI:
10.1186/s13287-018-1067-y. PMID:
30463587. PMCID:
PMC6249727.

6. Bozorgmehr M, Gurung S, Darzi S, Nikoo S, Kazemnejad S, Zarnani AH, Gargett CE. 2020; Endometrial and menstrual blood mesenchymal stem/stromal cells: biological properties and clinical application. Front Cell Dev Biol. 8:497. DOI:
10.3389/fcell.2020.00497. PMID:
32742977. PMCID:
PMC7364758.

7. Chang JH, Au HK, Lee WC, Chi CC, Ling TY, Wang LM, Kao SH, Huang YH, Tzeng CR. 2013; Expression of the pluripotent transcription factor OCT4 promotes cell migration in endometriosis. Fertil Steril. 99:1332–9.e5. DOI:
10.1016/j.fertnstert.2012.11.033. PMID:
23290742.

8. Deldar Y, Zarghami F, Pilehvar-Soltanahmadi Y, Dadashpour M, Zarghami N. 2017; Antioxidant effects of chrysin-loaded electrospun nanofibrous mats on proliferation and stemness preservation of human adipose-derived stem cells. Cell Tissue Bank. 18:475–87. DOI:
10.1007/s10561-017-9654-1. PMID:
28808812.

9. Fakih H, Baggett B, Holtz G, Tsang KY, Lee JC, Williamson HO. 1987; Interleukin-1: a possible role in the infertility associated with endometriosis. Fertil Steril. 47:213–7. DOI:
10.1016/S0015-0282(16)49993-0.

10. Banu SK, Lee J, Speights VO Jr, Starzinski-Powitz A, Arosh JA. 2008; Cyclooxygenase-2 regulates survival, migration, and invasion of human endometriotic cells through multiple mechanisms. Endocrinology. 149:1180–9. DOI:
10.1210/en.2007-1168. PMID:
18039779.

11. Kyama CM, Overbergh L, Debrock S, Valckx D, Vander Perre S, Meuleman C, Mihalyi A, Mwenda JM, Mathieu C, D'Hooghe TM. 2006; Increased peritoneal and endometrial gene expression of biologically relevant cytokines and growth factors during the menstrual phase in women with endometriosis. Fertil Steril. 85:1667–75. DOI:
10.1016/j.fertnstert.2005.11.060. PMID:
16759923.

12. Wu MH, Hsiao KY, Tsai SJ. 2015; Endometriosis and possible inflammation markers. Gynecol Minim Invasive Ther. 4:61–7. DOI:
10.1016/j.gmit.2015.05.001.

13. Proestling K, Birner P, Balendran S, Nirtl N, Marton E, Yerlikaya G, Kuessel L, Reischer T, Wenzl R, Streubel B, Husslein H. 2016; Enhanced expression of the stemness-related factors OCT4, SOX15 and TWIST1 in ectopic endometrium of endometriosis patients. Reprod Biol Endocrinol. 14:81. DOI:
10.1186/s12958-016-0215-4. PMID:
27881125. PMCID:
PMC5122168.

14. Song Y, Xiao L, Fu J, Huang W, Wang Q, Zhang X, Yang S. 2014; Increased expression of the pluripotency markers sex-determining region Y-box 2 and Nanog homeobox in ovarian endometriosis. Reprod Biol Endocrinol. 12:42. DOI:
10.1186/1477-7827-12-42. PMID:
24884521. PMCID:
PMC4031377.

15. Park JH, Daheron L, Kantarci S, Lee BS, Teixeira JM. 2011; Human endometrial cells express elevated levels of pluripotent factors and are more amenable to reprogramming into induced pluripotent stem cells. Endocrinology. 152:1080–9. DOI:
10.1210/en.2010-1072. PMID:
21209016. PMCID:
PMC3198966.

16. Siu MK, Wong ES, Kong DS, Chan HY, Jiang L, Wong OG, Lam EW, Chan KK, Ngan HY, Le XF, Cheung AN. 2013; Stem cell transcription factor NANOG controls cell migration and invasion via dysregulation of E-cadherin and FoxJ1 and contributes to adverse clinical outcome in ovarian cancers. Oncogene. 32:3500–9. DOI:
10.1038/onc.2012.363. PMID:
22945654.

17. Zhang W, Sui Y, Ni J, Yang T. 2016; Insights into the
Nanog gene: a propeller for stemness in primitive stem cells. Int J Biol Sci. 12:1372–81. DOI:
10.7150/ijbs.16349. PMID:
27877089. PMCID:
PMC5118783.
18. Forghanifard MM, Moghbeli M, Raeisossadati R, Tavassoli A, Mallak AJ, Boroumand-Noughabi S, Abbaszadegan MR. 2013; Role of SALL4 in the progression and metastasis of colorectal cancer. J Biomed Sci. 20:6. DOI:
10.1186/1423-0127-20-6. PMID:
23363002. PMCID:
PMC3599462.

19. Ardalan Khales S, Abbaszadegan MR, Abdollahi A, Raeisossadati R, Tousi MF, Forghanifard MM. 2015; SALL4 as a new biomarker for early colorectal cancers. J Cancer Res Clin Oncol. 141:229–35. DOI:
10.1007/s00432-014-1808-y. PMID:
25156818.

20. Joseph A, Baiju I, Bhat IA, Pandey S, Bharti M, Verma M, Pratap Singh A, Ansari MM, Chandra V, Saikumar G, Amarpal , Taru Sharma G. 2020; Mesenchymal stem cell-conditioned media: a novel alternative of stem cell therapy for quality wound healing. J Cell Physiol. 235:5555–69. DOI:
10.1002/jcp.29486. PMID:
31960454.

21. Noverina R, Widowati W, Ayuningtyas W, Kurniawan D, Afifah E, Laksmitawati DR, Rinendyaputri R, Rilianawati R, Faried A, Bachtiar I, Wirakusumah FF. 2019; Growth factors profile in conditioned medium human adipose tissue-derived mesenchymal stem cells (CM-hATMSCs). Clin Nutr Exp. 24:34–44. DOI:
10.1016/j.yclnex.2019.01.002.

22. Sefati N, Norouzian M, Abbaszadeh HA, Abdollahifar MA, Amini A, Bagheri M, Aryan A, Fadaei Fathabady F. 2018; Effects of bone marrow mesenchymal stem cells-conditioned medium on tibial partial osteotomy model of fracture healing in hypothyroidism rats. Iran Biomed J. 22:90–8.
23. Gauthaman K, Fong CY, Arularasu S, Subramanian A, Biswas A, Choolani M, Bongso A. 2013; Human Wharton's jelly stem cell conditioned medium and cell-free lysate inhibit human osteosarcoma and mammary carcinoma cell growth
in vitro and in xenograft mice. J Cell Biochem. 114:366–77. DOI:
10.1002/jcb.24367. PMID:
22930595.
24. Gauthaman K, Fong CY, Suganya CA, Subramanian A, Biswas A, Choolani M, Bongso A. 2012; Extra-embryonic human Wharton's jelly stem cells do not induce tumorigenesis, unlike human embryonic stem cells. Reprod Biomed Online. 24:235–46. DOI:
10.1016/j.rbmo.2011.10.007. PMID:
22196893.

25. Nikoo S, Ebtekar M, Jeddi-Tehrani M, Shervin A, Bozorgmehr M, Vafaei S, Kazemnejad S, Zarnani AH. 2014; Menstrual blood-derived stromal stem cells from women with and without endometriosis reveal different phenotypic and functional characteristics. Mol Hum Reprod. 20:905–18. DOI:
10.1093/molehr/gau044. PMID:
24939730.

26. Chalpe A, Law C, Dumdie J, Hansen K, Eyster K. 2015; TNFα and IL1β stimulate differential gene expression in endometrial stromal cells. Adv Biol Chem. 5:126–41. DOI:
10.4236/abc.2015.52010.
27. Ota H, Igarashi S, Sasaki M, Tanaka T. 2001; Distribution of cyclooxygenase-2 in eutopic and ectopic endometrium in endometriosis and adenomyosis. Hum Reprod. 16:561–6. DOI:
10.1093/humrep/16.3.561. PMID:
11228229.

28. Matsuzaki S, Canis M, Pouly JL, Wattiez A, Okamura K, Mage G. 2004; Cyclooxygenase-2 expression in deep endometriosis and matched eutopic endometrium. Fertil Steril. 82:1309–15. DOI:
10.1016/j.fertnstert.2004.03.059. PMID:
15533352.

29. Nickoloff BJ, Qin JZ, Chaturvedi V, Denning MF, Bonish B, Miele L. 2002; Jagged-1 mediated activation of notch signaling induces complete maturation of human keratinocytes through NF-kappaB and PPARgamma. Cell Death Differ. 9:842–55. DOI:
10.1038/sj.cdd.4401036. PMID:
12107827.

30. Santamaria X, Massasa EE, Taylor HS. 2012; Migration of cells from experimental endometriosis to the uterine endometrium. Endocrinology. 153:5566–74. DOI:
10.1210/en.2012-1202. PMID:
22968642. PMCID:
PMC3473215.

31. Liu L, Zhang J, Yang X, Fang C, Xu H, Xi X. 2015; SALL4 as an epithelial-mesenchymal transition and drug resistance inducer through the regulation of c-Myc in endometrial cancer. PLoS One. 10:e0138515. DOI:
10.1371/journal.pone.0138515. PMID:
26407074. PMCID:
PMC4583418.

32. Lin SC, Li YH, Wu MH, Chang YF, Lee DK, Tsai SY, Tsai MJ, Tsai SJ. 2014; Suppression of COUP-TFII by proinflammatory cytokines contributes to the pathogenesis of endometriosis. J Clin Endocrinol Metab. 99:E427–37. DOI:
10.1210/jc.2013-3717. PMID:
24423359. PMCID:
PMC5393480.

33. Götte M, Wolf M, Staebler A, Buchweitz O, Kiesel L, Schüring AN. 2011; Aberrant expression of the pluripotency marker SOX-2 in endometriosis. Fertil Steril. 95:338–41. DOI:
10.1016/j.fertnstert.2010.08.006. PMID:
20850729.

34. Zhu C, Yu J, Pan Q, Yang J, Hao G, Wang Y, Li L, Cao H. 2016; Hypoxia-inducible factor-2 alpha promotes the proliferation of human placenta-derived mesenchymal stem cells through the MAPK/ERK signaling pathway. Sci Rep. 6:35489. DOI:
10.1038/srep35489. PMID:
27765951. PMCID:
PMC5073233.

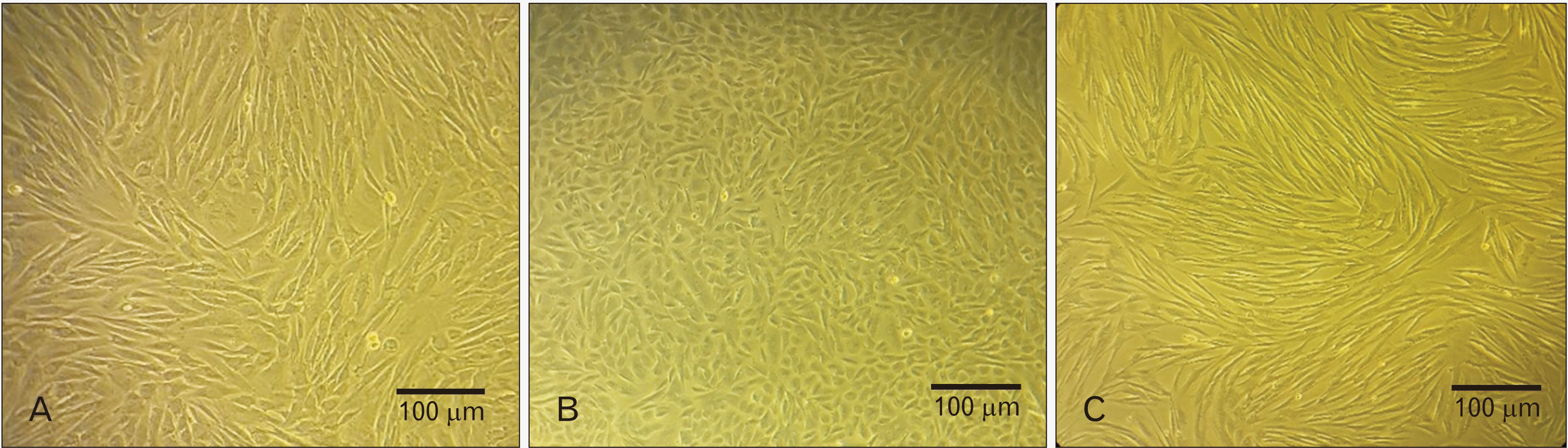
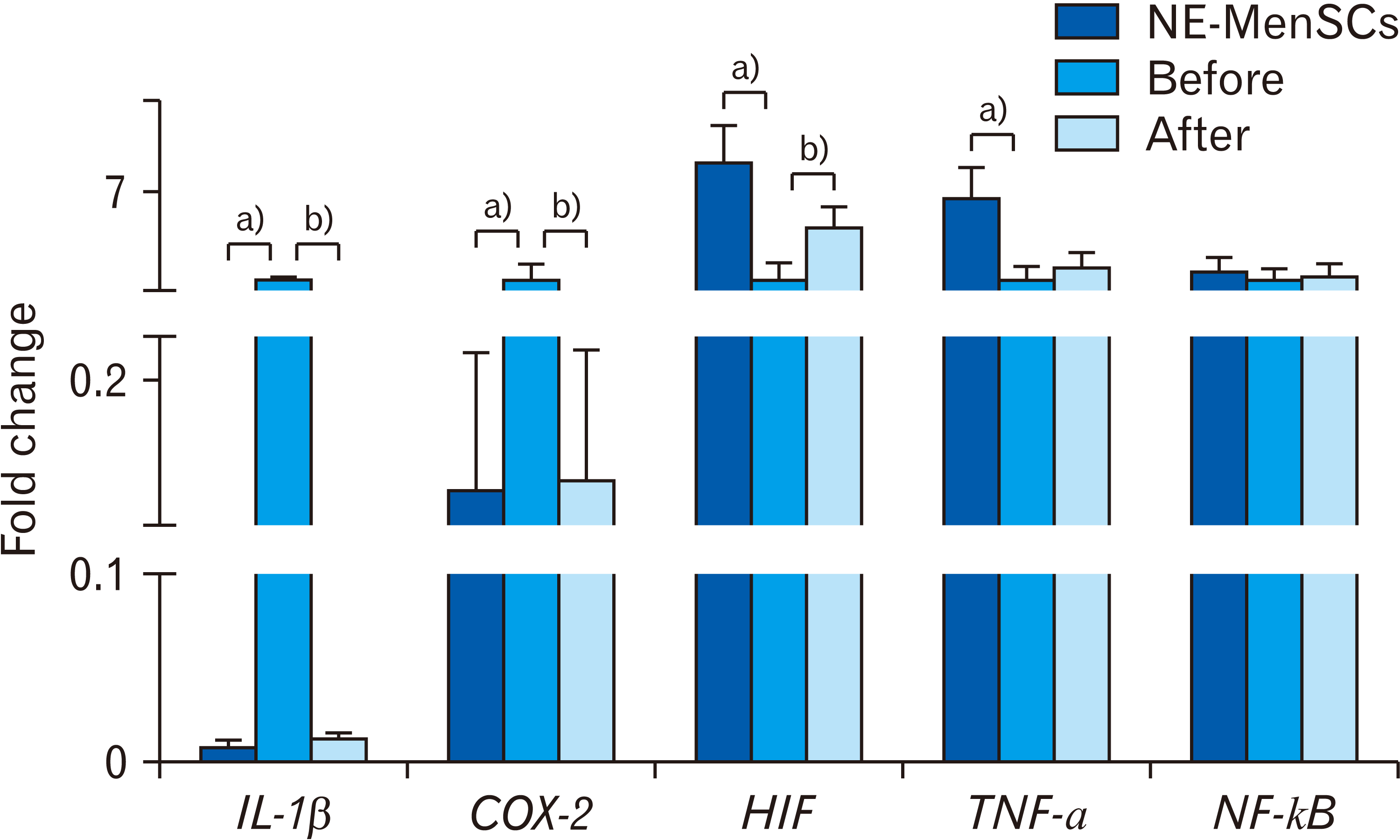
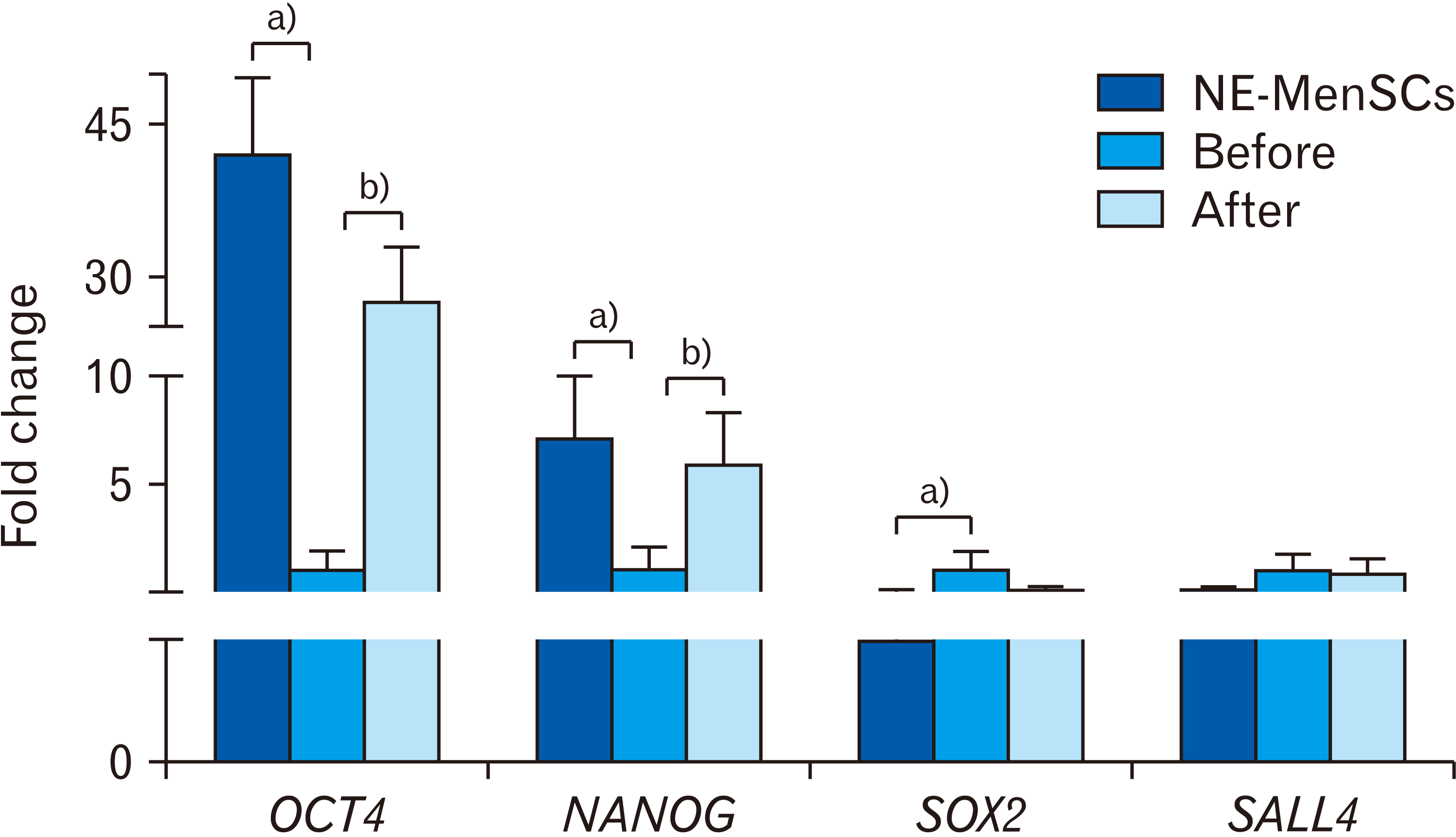




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download