INTRODUCTION
Anterior open bite is a malocclusion characterized by the lack of contact between the dental arches in the anterior region.
1 This condition harms the oral health-related quality of life of children,
2 unbalances the stomatognathic system,
3 and has a multifactorial origin involving a combination of skeletal, dental, and soft tissue factors.
4
Open bite can be associated with the breakdown of the peribuccal and intraoral muscle balance caused by deleterious oral habits such as prolonged digital and pacifier sucking, which results in tongue thrusting at rest and during swallowing
1,4,5 From the age of 5 years, orthodontic interception is necessary because lingual interposition between the incisors is a harmful factor that prolongs malocclusion.
4,6-8 The treatment primarily targets elimination of the harmful habit and lingual interposition.
4 There are different ways to perform such interventions; one of them involves the use of an orthodontic accessory called a spur, which is a passive orthodontic appliance. The primary purpose of spurs is backward repositioning of the tongue, thus avoiding tongue interposition between the mandibular and maxillary incisors.
9
Interleukins (ILs) mediate the mechanical effect of orthodontic movement in the periodontal ligament (PDL). These biological markers produced by inflammatory, bone, and PDL cells can be measured in the gingival crevicular fluid (GCF).
10,11 The presence of ILs in GCF is a valuable marker for monitoring the response of teeth to orthodontic treatment, because it reflects the microenvironment where forces are exerted.
12,13
Studies have observed differences in the concentration of ILs such as IL-1β, IL-6, and tumor necrosis factor-alpha (TNF-α) in GCF during active orthodontic movement.
12-19 However, there is scarce information concerning the presence of biological markers during the initial periods of movement with passive orthodontic treatment. The purpose of the present study was to evaluate the periodontal conditions and the response of GCF cytokine markers during passive orthodontic treatment with spurs in children with anterior open bite. The hypothesis was that passive appliances could interfere with clinical and periodontal parameters and increase biomarker expression.
MATERIALS AND METHODS
This longitudinal clinical trial research was approved by the Research Ethics Committee of the institution (CAAE: 87714218.0.0000.5149) with a duration of two-year period. All subjects provided written informed consent to participate in the study. The study was a group comprised 20 patients selected according to the following inclusion criteria: indications for interceptive orthodontic treatment, presence of anterior open bite, and good oral hygiene and periodontal health. Patients with systemic diseases, those who received treatment with antibiotics and anti-inflammatory drugs in the past 3 months, those with bleeding during GCF collection, and those with any orthodontic accessory were excluded.
Periodontal parameters, namely the probing depth, the percentage of visible plaque, and gingival bleeding, were recorded.
13 Clinical examination and device bonding were performed by a single trained and calibrated researcher (interexaminer kappa = 0.91 and intraexaminer kappa = 0.83). Seven days before the first day of sample collection, the participants received hygiene instructions and underwent cleaning of the dental and oral environment. Cumulative data indicate that there is an increase in GCF cytokines as early as 1 minute to 1 hour after device bonding, reaching a peak at 24 hours; this is followed by a decrease, with a new peak at day 7.
20 Thus, according to the procedures used in previous studies,
13,16,18,20 periodontal evaluation and GCF collection were performed before placement of the appliance (baseline) and within 24 hours and 7 days after the device was bonded.
Relative isolation was performed, phosphoric acid (Fusion-Duralink; Angelus, Londrina, Brazil) was applied for 30 seconds to the enamel surface, and the Transbond XT adhesive system kit (3M Unitek, Monrovia, CA, USA) was applied according to the manufacturer’s recommendations. Bonded spurs with a 2.8-mm-wide base (ref. number 30.60.002; Morelli, Sorocaba, SP, Brazil)
21 were installed in the central region of the lingual surface of the mandibular incisor; pressure was applied to the center of the accessory so that the excess composite resin could leak out from the sides of the spurs and be removed. Two 40-second photoactivation cycles, one on the lingual side and one on the incisal side, were performed with an LED lamp (1,000 mW/cm
2, Bluephase N; Ivoclar Vivadent Inc., Amherst, NY, USA) (
Figure 1).
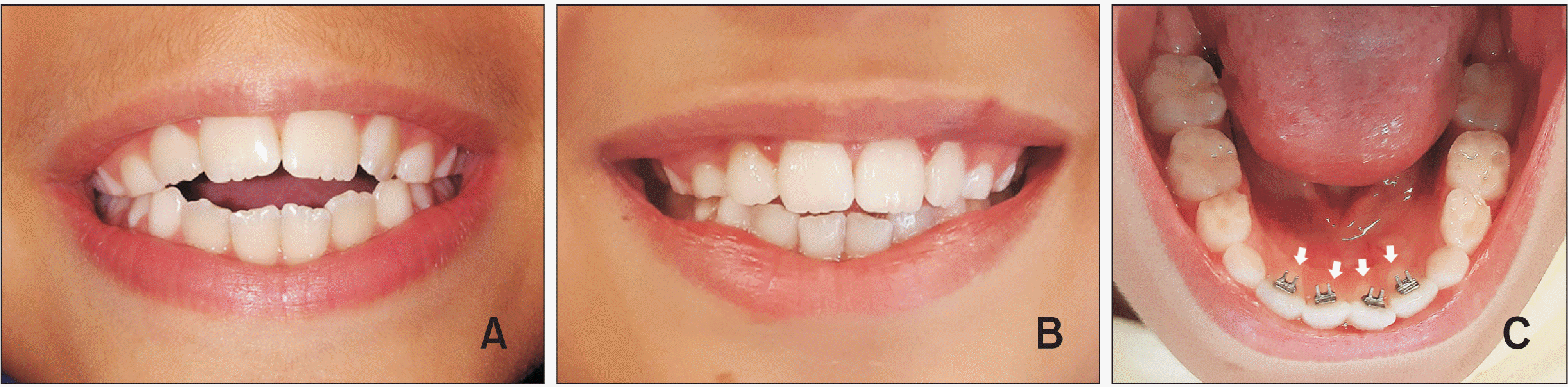
Figure 1
A, Representative image of an 8-year-old boy with anterior open bite. B, Removal of tongue interposition after spur bonding allowed teeth eruption and bone remodeling with an increase in cytokine levels. The same participant shows some correction of the malocclusion at 1 year after attachment of the spur. C, The spurs are attached to the central regions of the lingual surfaces of the mandibular incisors (white arrows indicate the cervical regions of the mandibular incisors).

GCF samples were collected from the four mandibular and maxillary central incisors. Before fluid collection, all clinically detectable biofilm was removed without touching the gingiva in order to minimize contamination of the absorbent paper filters with blood. Following isolation with a cotton roll, the gingiva was gently air-dried and GCF was collected using Periopaper
® strips (gingival fluid collection strips, ref. number 593520, Periopaper
®; Oraflow, Hewlett, NY, USA).
13 The tape was introduced into the gingival sulcus from the lingual side until it found tissue resistance, and it was held in place for 30 seconds. Samples visibly contaminated with blood were discarded. For better accuracy, GCF was quantified on the Periotron
® 8000 apparatus (Oraflow) immediately after each collection. The Periopaper
® strips were inserted into sterile vials, which were immersed in ice and stored at −80°C until further analysis. Periotron
® 8000 readings were converted to volume measurements in microliters using a standard human serum curve.
GCF samples were extracted in two steps. First, 25 µL of cytokine extraction solution (phosphate-buffered saline [0.4 mM NaCl, 10 mM NaPO
4]) containing protease inhibitors (0.1 mM phenylmethylsulfonylfluoride, 0.1 mM hydrochloric benzethonium, 10 mM ethylenediaminetetraacetic acid, and 0.01 mg/mL aprotinin A; pH 7.4) and Tween 20 (0.05%) was added to each Eppendorf tube containing the Periopaper
® strip. The tubes were shaken for 30 seconds and centrifuged for 10 minutes at 10,000 RPM at 4°C. The supernatant was removed and stored in a new Eppendorf tube, and the same process was repeated one more time. At the end, a total of 50 µL was obtained from each Periopaper
® strip. The supernatants were mixed in a single Eppendorf tube and shaken. The material of the maxillary and mandibular incisor groups was pooled for each collection time, and 50 µL of the supernatant was separated for cytometric bead array (CBA). The other samples were stored at −80°C.
13
GCF analysis was performed using a BDTM CBA Human Inflammatory Cytokines Human Kit (reference number 551811; BD Biosciences, San Diego, CA, USA) and a BD FACSCaliburTM flow cytometer (Becton Dickinson, San Jose, CA, USA) according to manufacturer’s guidelines. This kit can measure IL-8, IL-1β, IL-6, IL-10, TNF-α, and IL-12p70 in a single human sample. The cytokine concentrations in GCF were corrected by the total volume of fluid obtained, and the values were recorded in pg/mL.
Statistical analysis
The data were expressed as mean ± standard deviation. The datasets showed non-normal distribution when analyzed by the D'Agostino & Pearson omnibus normality test and the Shapiro–Wilk normality test. Differences between groups were analyzed using the nonparametric Wilcoxon test at each time point, and the difference between the mandibular and maxillary incisor groups was determined by the Mann–Whitney U test. Correlations were determined by Pearson’s correlation test. The level of significance was set at P < 0.05 in all analyses.
DISCUSSION
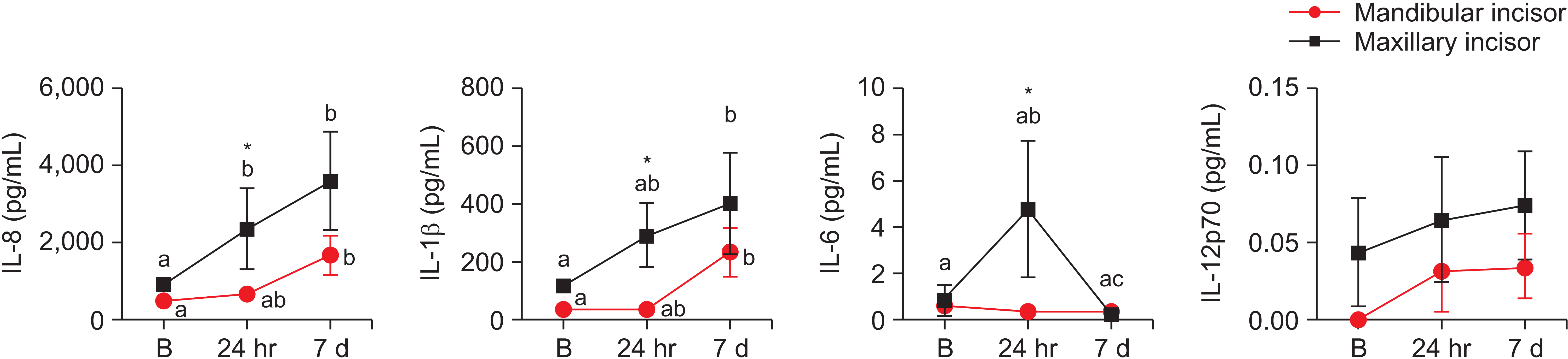
Studies demonstrating the effects of passive orthodontic appliances for open bite correction in children are lacking. In the present study, we analyzed the clinical, periodontal, and molecular effects of a passive orthodontic appliance on the mandibular and maxillary incisors at 24 hours and 7 days after bonding. The major findings included increased gingival bleeding after spur attachment and time point fluctuations in the production of IL-8, IL-1β, and IL-6. Cytokine levels in GCF were higher in the maxillary group. Other than the GCF volume, none of the clinical data were correlated with the levels of the analyzed ILs.
The use of orthodontic appliances attached to the teeth is associated with changes in clinical parameters; therefore, periodontal inflammation should be considered during treatment.
15,17,22 Our findings showed that the increase in gingival bleeding at 7 days after orthodontic spur bonding to the mandibular incisors was correlated with an increased dental plaque percentage. This result is consistent with that in the study conducted by Pejda et al.,
23 who reported a significant increase in gingival bleeding in patients treated with a fixed appliance after bracket bonding. Another study also agrees with these findings by demonstrating that the presence of orthodontic accessories contributes to the worsening of periodontal clinical parameters, such as the plaque percentage, thus resulting in increased inflammation and gingival bleeding.
24 These changes are directly related to difficulties in cleaning and consequent accumulation of a dental biofilm,
17,22 and they may persist until the end of orthodontic treatment, when the accessories are removed.
22 Moreover, in the present study, an increased dental plaque percentage was correlated with an increased GCF volume at 24 hours after spur attachment. Similar results have been reported by Grant et al.,
17 who demonstrated a positive correlation between plaque accumulation and the GCF volume at sites of orthodontic force application.
15,24
The release of pro-inflammatory markers may be associated with an increase in dental plaque in patients with orthodontic appliances.
17 An increase in the GCF volume has also been considered an indicator of periodontal inflammation and is related to an increase in IL levels.
25 However, no correlation was detected between clinical data other than the GCF volume and the GCF cytokine levels analyzed in this study. First, IL was not correlated with periodontal parameters; this indicates that IL expression in the maxillary and mandibular groups was associated with tooth movement and open bite correction. Second, IL was significantly higher for the maxillary than for the mandibular teeth, which were the sites of spur attachment. Therefore, we can conclude that the increased cytokine levels in GCF were not a result of spur-induced gingival/periodontal inflammation; rather, they were associated with tooth movement during open bite correction with the passive orthodontic appliance.
Anterior open bite correction with spurs is considered to involve passive movement,
9 and the elimination of harmful oral habits allows correct positioning of the tongue and permits the peribuccal muscles to exert force on the teeth,
4,6 with induction of bone remodeling and cytokine release.
25 While most investigators focus on active treatments, in the present study, we evaluated the production of biomarkers in GCF during the early periods of passive orthodontic tooth movement.
Various types of inflammatory mediators are involved in the cascade of biological events. Biomarkers are present in GCF during orthodontic tooth movement because of induction of bone remodeling caused by the forces applied to the teeth.
11,12,18 The decision to study the cytokines reported in the present study was based on literature data showing the kinetics of these molecules in GCF after mechanical force application.
11,12,18 GCF analysis was performed using the BD
TM CBA Human Inflammatory Cytokines Kit, which can measure bone remodeling markers such as IL-8,
12 IL-1β,
16,17 IL-6,
13,18 IL-10,
20 TNF-α,
17,26 and IL-12p70
26 in a single human sample.
Biomarkers are present in GCF during active orthodontic movement due to the forces applied to the teeth.
11,12,18 Teixeira et al.
19 identified an increase in the levels of 32 different types of cytokines/cytokine receptors in an animal model over a 24-hour period of orthodontic force application; among these cytokines, IL-3, IL-18, TNF-a, IL-1β, and IL-6 showed remarkable increases. Similar results were obtained by Tuncer et al.,
12 who reported that active orthodontic movement induced an increase in the release of IL-8 in GCF at 24 hours and 6 days in adults. In the present study, a passive orthodontic model was used, and likewise, it was possible to identify a significant increase in IL-8, IL-6, and IL-1β. Of note, although greater exudation of GCF is related to clinical and periodontal parameters, it does not explain the increase in IL levels after passive appliance bonding, when the cytokine levels are normalized by the amount of GCF volume in each sample.
In this investigation, IL-6 was increased only in the maxillary incisor group at 24 hours after spur bonding, with a remarkable decrease at 7 days. This result is probably related to the role of IL-6 in the initial moments of orthodontic tooth movement.
18 Other studies with force application through active movement have also detected the presence of IL-1β during the initial phase of orthodontic treatment.
16,17,19,27,28 In the present study, IL-1β values were significant only at the 7-day time point in both the mandibular and maxillary groups, with higher levels in the maxillary than in the mandibular group at 24 hours after spur attachment. However, it is necessary to consider that the amount of force applied to the teeth and the speed of tooth movement are directly related to the amount of ILs released,
17,27,28 and a passive appliance was used in the present study. The elimination of tongue interposition after spur bonding might facilitate tooth eruption and bone remodeling via these molecules for the correction of open bite in children.
29
In contrast to the approach used in other studies,
14,15 a lingual approach was used for GCF sample collection in the present study for two main reasons. First, the lingual area was the area closest to the site of spur attachment, so sampling from this site would allow accurate analysis of clinical parameters and biomarkers in GCF. Second, in a manner analogous to that in the study of Uematsu et al.,
18 wherein the canines were moved in the distal direction and GCF was collected from the distal side of the teeth, spur attachment was supposed to move the maxillary and mandibular incisors in the lingual direction in the present study.
21,30 Moreover, Aliaga-Del Castillo et al.
21 and Canuto et al.
30 used superimposition of cephalometric tracings to demonstrate reduced labial tipping and protrusion of the maxillary incisors and increased labial inclination of the mandibular incisors after orthodontic treatment with a spur. In summary, these findings indicate that the maxillary and mandibular central incisors are the teeth affected after spur bonding; this justifies the selection of the 4 maxillary and mandibular central incisors for GCF sample collection in the present study.
Different devices can be used for early anterior open bite correction, and most of them promote dental changes, especially in the anterior region, and contribute to anterior bite reduction.
21 In the present study, lingual spurs were used,
8 and it was well accepted by the children. Without the presence of harmful habits and tongue interposition, the musculature of the lips exerts a force on the vestibular surface of the incisors, stimulating bone remodeling and tooth eruption for open bite correction.
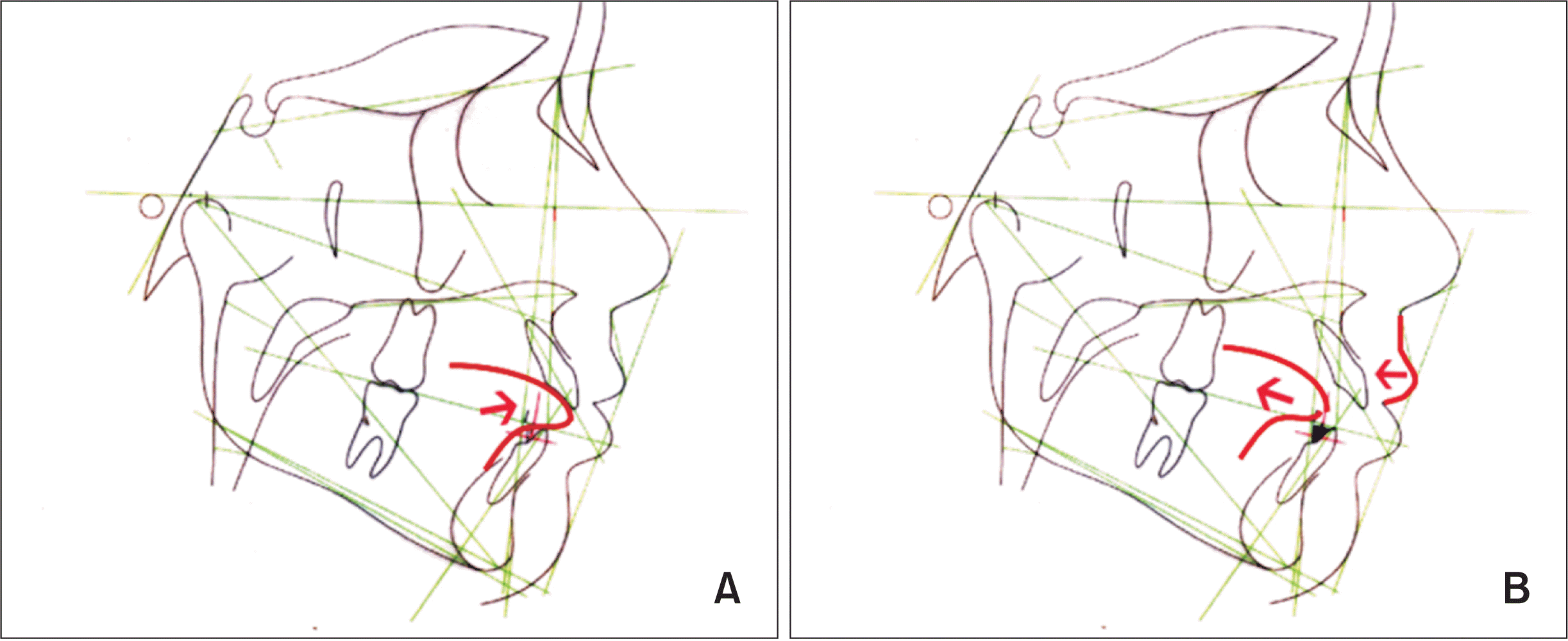
3,5,6 After spur attachment, the interposed tongue placed in an interdental resting posture (
Figure 3A) is forced to a backward position (
Figure 3B).
29 Generally, patients with open bite also exhibit open lip relationships and impaired tongue positions.
4,8 In subjects with an open lip and open bite, the lip pressure is lower than the tongue interposition pressure.
4 The present study demonstrated that spur bonding prevented lingual interposition, possibly resulting in increased lip pressure on the maxillary incisors in the initial period and thus activating alveolar bone remodeling and incisor eruption with an increase in GCF IL levels at 24 hours and 7 days.
Figure 3
A, Representative image of tongue interposition between the mandibular and maxillary incisors in a child with anterior open bite. B, After spur attachment (black triangles bonded to the lingual surface of the mandibular incisors), the tongue moved backward, and the upper lip exerted pressure on the central incisors with effects on alveolar bone remodeling and tooth eruption at 24 hours after the attachment.

Most clinical studies involving the analysis of IL levels in GCF have been conducted on teenagers or adults receiving treatment with fixed orthodontic appliances.
12,15,16,18,27,28 To the best of our knowledge, this is the first study to demonstrate the molecular mechanisms involved in open bite correction using spur attachment in children. The data suggest that GCF ILs play a role in tooth eruption and bone remodeling after the attachment of passive orthodontic appliances in children with open bite. However, the study was limited by the fact that the analysis of GCF cytokines was limited to the maxillary and mandibular central incisors. In further studies, additional analysis of the maxillary and mandibular lateral incisors would provide important information. Moreover, GCF collection from different sites such as the buccal surface of the maxillary and mandibular central incisors should be implemented in future studies.






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download