TO THE EDITOR: Dasatinib, a second-generation tyrosine kinase inhibitor (TKI), targets many tyrosine kinases, including Src and ABL kinases, whereas imatinib targets only BCR-ABL1. Therefore, dasatinib could be used in the treatment of BCR-ABL1+ chronic myeloid leukemia and BCR-ABL1+ acute lymphoblastic leukemia [1, 2]. In addition, dasatinib inhibits most mutated forms of BCR-ABL1; it can be an effective option for patients who are refractory to imatinib [3]. Pulmonary arterial hypertension (PAH) is one of the side effects of dasatinib: 0.45–5% of patients who received dasatinib developed PAH in a previous study involving adult populations [4, 5]. As the indications of dasatinib have been extended to pediatric patients, there are growing concerns about dasatinib-induced PAH [6]. To the best of our knowledge, this is the first case of dasatinib-induced PAH in a pediatric patient with BCR/ABL1+ lymphoblastic lymphoma from chronic myeloid leukemia (CML). The Institutional Review Board of the Seoul National University Hospital approved the procedure of reviewing medical records, and the requirement for written consent was waived (H-2002-125-1104).
A 4-year-old girl developed BCR-ABL1+ lymphoblastic lymphoma in the nasal cavity after receiving imatinib for 15 months for CML. At diagnosis of CML, BCR-ABL1 fusion was detected in 94% of cells on the initial bone marrow exam with fluorescence in situ hybridization (FISH). Translocation t(9;22)(q34;q11) was found by multiplex nested reverse transcriptase-polymerase chain reaction (RT-PCR) of bone marrow aspirate and peripheral blood. The initial BCR-ABL1 major International Scale (IS), used for the measurement of residual disease, was 56.4% on bone marrow aspirate. A year and three months after the initiation of imatinib, BCR-ABL1 major IS dropped to 0.1% on peripheral blood. However, a diagnosis of BCR-ABL1+ lymphoblastic lymphoma was made after a soft tissue mass was found in the nasal cavity 17 months after diagnosis of CML: BCR-ABL1 fusion was identified in 99% of cells on endoscopic biopsy and 6.3% of cells on bone marrow aspirate. The diagnosis was made through FISH analysis; real-time quantitative polymerase chain reaction (RQ-PCR) was not done. The patient remained in remission per the bone marrow exam results, without any evidence of lymphoma involvement or exacerbation of CML. The regimen of induction chemotherapy included prednisolone, vincristine, daunorubicin, L-asparaginase, cyclophosphamide, and intrathecal chemotherapy. After induction chemotherapy, bone marrow results showed complete response, with BCR-ABL1 fusion detected in 0.3% of cells by FISH and a BCR-ABL1 major IS ratio of 0.07, which was below 0.1. Dasatinib was added, and allogeneic hematopoietic stem cell transplantation (HSCT) from a matched unrelated donor was performed 6 months after diagnosis of BCR-ABL1+ lymphoblastic lymphoma, with busulfan, fludarabine, and etoposide as the conditioning regimen [7]. The last BCR-ABL1 major IS before HSCT was 0.01%, and it went below the detection limit after HSCT. Dasatinib was resumed 3 months after HSCT. One year after HSCT, the patient developed exertional dyspnea. Chest computed tomography (CT) showed mosaic attenuation in both lungs. The dyspnea got worse and resulted in the limitation of ordinary activity to 10 minutes. The dose of dasatinib was then decreased from 70 mg to 60 mg due to persistent moderate neutropenia.
Seven months after the initial symptoms of dyspnea and 21 months after HSCT, the patient presented with fever, cough, tachypnea, and poor oral intake with lethargy. The symptoms persisted, even after management with peramivir for influenza. Further chest CT evaluation was performed to rule out the possibility of bronchopneumonia, and empirical antibiotics were administered. Even though the fever subsided, the patient rapidly deteriorated into shock and required inotropics. Dobutamine and low-dose dopamine were administered.
After echocardiographic assessment, the patient was diagnosed with mild-to-moderate secondary PAH. Enlargement of the right ventricle (RV) and right atrium (RA) with mild tricuspid regurgitation, as well as a D-shaped intraventricular septum (IVS), were observed. The pressure gradient between the RV and RA was 47 mmHg, and posterior wall motion was decreased, implying that RV function was decreased. Sildenafil was administered. The tachypnea slowly subsided, but the patient remained with persistent tachycardia and mild respiratory distress. Low-dose dopamine was discontinued the following day and dobutamine was tapered off 6 days after initiation. Simultaneously, digoxin was started and continued for approximately a month. Dasatinib was discontinued to rule out dasatinib-induced secondary PAH, although no previous case has been reported in children. After the discontinuation of dasatinib, monthly echocardiography showed improvement of clinical markers, including IVS configuration, pulmonary artery pressure, and tricuspid valve regurgitation (TR), with the values eventually approaching the normal ranges (Fig. 1, Table 1) [8]. Oxygen supply through flow-by was tapered off completely after 3 days. The tachypnea and tachycardia improved after 11 days from the discontinuation of dasatinib. BCR-ABL1 rearrangement had remained below the detectable limit. The patient had sustained remission off dasatinib until the last follow-up, with the last BCR-ABL1 major IS kept negative; this was 4.2 years after HSCT.
A retrospective review of previous chest radiographs revealed cardiomegaly and prominent pulmonary vessel opacity, suggesting that the PAH presented about 15 months after HSCT. This change became prominent when the patient developed influenza viral pneumonia 20 months after HSCT. The hilar-to-thoracic index, which is known to be correlated with PAH [9], gradually increased during dasatinib therapy (Fig. 2). To evaluate the hilar-to-thoracic index, the horizontal distance from the midline to the first division of the pulmonary arteries on both sides was summed. The sum was divided by the maximum transverse diameter of the thoracic cage [10].
In the present case, the diagnosis of dasatinib-induced PAH was confirmed 25 months after the initiation of dasatinib (Fig. 2), and the PAH symptoms developed after 18 months of dasatinib therapy. There were suggestive radiologic changes in the chest radiographs. From the initiation of drug therapy, it took 16 months for the initial symptom of exertional dyspnea to develop. In the adult population, the median duration between the initiation of dasatinib and the diagnosis of dasatinib-induced PAH was reportedly 34 months (range, 8–48 mo). The French registry showed early development of PAH in patients with BCR-ABL1+ acute lymphoblastic leukemia, with a median value of 8 months after the initiation of dasatinib [4].
Notably, adults with right heart dysfunction due to PAH easily develop symptoms, whereas pediatric patients adjust cardiac output and become symptomatic only after over-exertion [11]. It can be inferred that echocardiographic changes may precede fatal symptoms of right heart dysfunction in the pediatric population, considering the delayed onset of disease and the indolent progression in children.
Dasatinib-induced PAH can be irreversible if fatal events, such as right heart dysfunction, occur. Reportedly, a few cases of mortality and morbidity due to an irreversible course of severe dasatinib-induced PAH in adults have been documented [4]. In the present case, after the discontinuation of dasatinib, more than 3 months elapsed before improvements in clinical parameters were observed, suggesting a substantially chronic course. Further time might be needed for the patient to completely recover.
Moreover, we witnessed that children are not safe from dasatinib-induced PAH. Additionally, we demonstrated that dasatinib-induced PAH is reversible in this patient population if the drug is discontinued before the onset of the fatal course of right heart dysfunction. Considering the different disease courses observed, there remains a crucial need to accumulate cases in pediatric patients to assist in the development of a clinical protocol for early detection [4].
The drug interactions of dasatinib should also be thoroughly reviewed. Tacrolimus for graft-versus-host disease had also been used after HSCT until a diagnosis of PAH. As an inhibitor of P-glycoprotein (Pgp), tacrolimus increases dasatinib exposure, which, in turn, acts as an inhibitor of CYP3A4, increasing exposure of tacrolimus. Considering the interaction between tacrolimus and dasatinib, tacrolimus should be used with caution in patients concomitantly receiving dasatinib [12].
There is a need for noninvasive tools of PAH evaluation since diagnostic right heart catheterization is extremely invasive. In this case, chest X-ray and hilar-to-thoracic index were considered markers as they could be quantified. Furthermore, the hilar-to-thoracic index was well correlated with the progression of PAH. The hilar-to-thoracic index could provide an effective method to detect the early stages of PAH and be a basis for deciding echocardiographic evaluation, necessitating further evidence to be generalized as routine. Appropriate echocardiography follow-up can help prevent delayed diagnosis of dasatinib-induced PAH, which can lead to irreversible right heart dysfunction. Further studies are needed to evaluate the benefits and risks of noninvasive PAH evaluation during dasatinib treatment and to eventually prevent silent progression due to delayed detection of reversible secondary PAH.
Acknowledgments
This study was supported by a grant from the Ministry of Food and Drug Safety in 2020 (20182MFDS443).
REFERENCES
1. Baccarani M, Cortes J, Pane F, et al. 2009; Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 27:6041–51. DOI: 10.1200/JCO.2009.25.0779. PMID: 19884523. PMCID: PMC4979100.

2. McCafferty EH, Dhillon S, Deeks ED. 2018; Dasatinib: a review in pediatric chronic myeloid leukemia. Paediatr Drugs. 20:593–600. DOI: 10.1007/s40272-018-0319-8. PMID: 30465234.

3. Baccarani M, Deininger MW, Rosti G, et al. 2013; European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 122:872–84. DOI: 10.1182/blood-2013-05-501569. PMID: 23803709. PMCID: PMC4915804.
4. Montani D, Bergot E, Günther S, et al. 2012; Pulmonary arterial hypertension in patients treated by dasatinib. Circulation. 125:2128–37. DOI: 10.1161/CIRCULATIONAHA.111.079921. PMID: 22451584.

5. El-Dabh A, Acharya D. 2019; EXPRESS: pulmonary hypertension with dasatinib and other tyrosine kinase inhibitors. Pulm Circ. 9:2045894019865704. DOI: 10.1177/2045894019865704. PMID: 31274047. PMCID: PMC6664660. PMID: df5016ddf59f4a5abdca670e34588af5.
6. Gore L, Kearns PR, de Martino ML, et al. 2018; Dasatinib in pediatric patients with chronic myeloid leukemia in chronic phase: results from a phase II trial. J Clin Oncol. 36:1330–8. DOI: 10.1200/JCO.2017.75.9597. PMID: 29498925. PMCID: PMC5929218.

7. Lee JW, Kang HJ, Kim S, et al. 2015; Favorable outcome of hematopoietic stem cell transplantation using a targeted once-daily intravenous busulfan-fludarabine-etoposide regimen in pediatric and infant acute lymphoblastic leukemia patients. Biol Blood Marrow Transplant. 21:190–5. DOI: 10.1016/j.bbmt.2014.09.013. PMID: 25255163.
8. Shimada R, Takeshita A, Nakamura M. 1984; Noninvasive assessment of right ventricular systolic pressure in atrial septal defect: analysis of the end-systolic configuration of the ventricular septum by two-dimensional echocardiography. Am J Cardiol. 53:1117–23. DOI: 10.1016/0002-9149(84)90647-7. PMID: 6702690.

9. Altschul E, Remy-Jardin M, Machnicki S, et al. 2019; Imaging of pulmonary hypertension: pictorial essay. Chest. 156:211–27. DOI: 10.1016/j.chest.2019.04.003. PMID: 30981724.
10. Lupi E, Dumont C, Tejada VM, Horwitz S, Galland F. 1975; A radiologic index of pulmonary arterial hypertension. Chest. 68:28–31. DOI: 10.1378/chest.68.1.28. PMID: 1149525.

11. Barst RJ, Ertel SI, Beghetti M, Ivy DD. 2011; Pulmonary arterial hypertension: a comparison between children and adults. Eur Respir J. 37:665–77. DOI: 10.1183/09031936.00056110. PMID: 21357924. PMCID: PMC3128436.

12. Haouala A, Widmer N, Duchosal MA, Montemurro M, Buclin T, Decosterd LA. 2011; Drug interactions with the tyrosine kinase inhibitors imatinib, dasatinib, and nilotinib. Blood. 117:e75–87. DOI: 10.1182/blood-2010-07-294330. PMID: 20810928.

Fig. 1
Echocardiographic changes after discontinuation of dasatinib. The interventricular septum is marked with arrows. At diagnosis of pulmonary arterial hypertension, echocardiography showed mild tricuspid regurgitation (TR) with a velocity of 3.4 m/sec and a D-type interventricular septum (IVS). The estimated RV to RA pressure gradient was 47 mmHg (A) [8]. Only trivial TR with a velocity of 2.7–2.8 m/sec remained and the shape of the IVS improved to B-type a month after the discontinu-ation of dasatinib (B). After 3 months, the IVS was still flat (C). It recovered to almost normal 6 months after discontinuation of dasatinib, and the TR velocity was 1.5 m/sec (D).
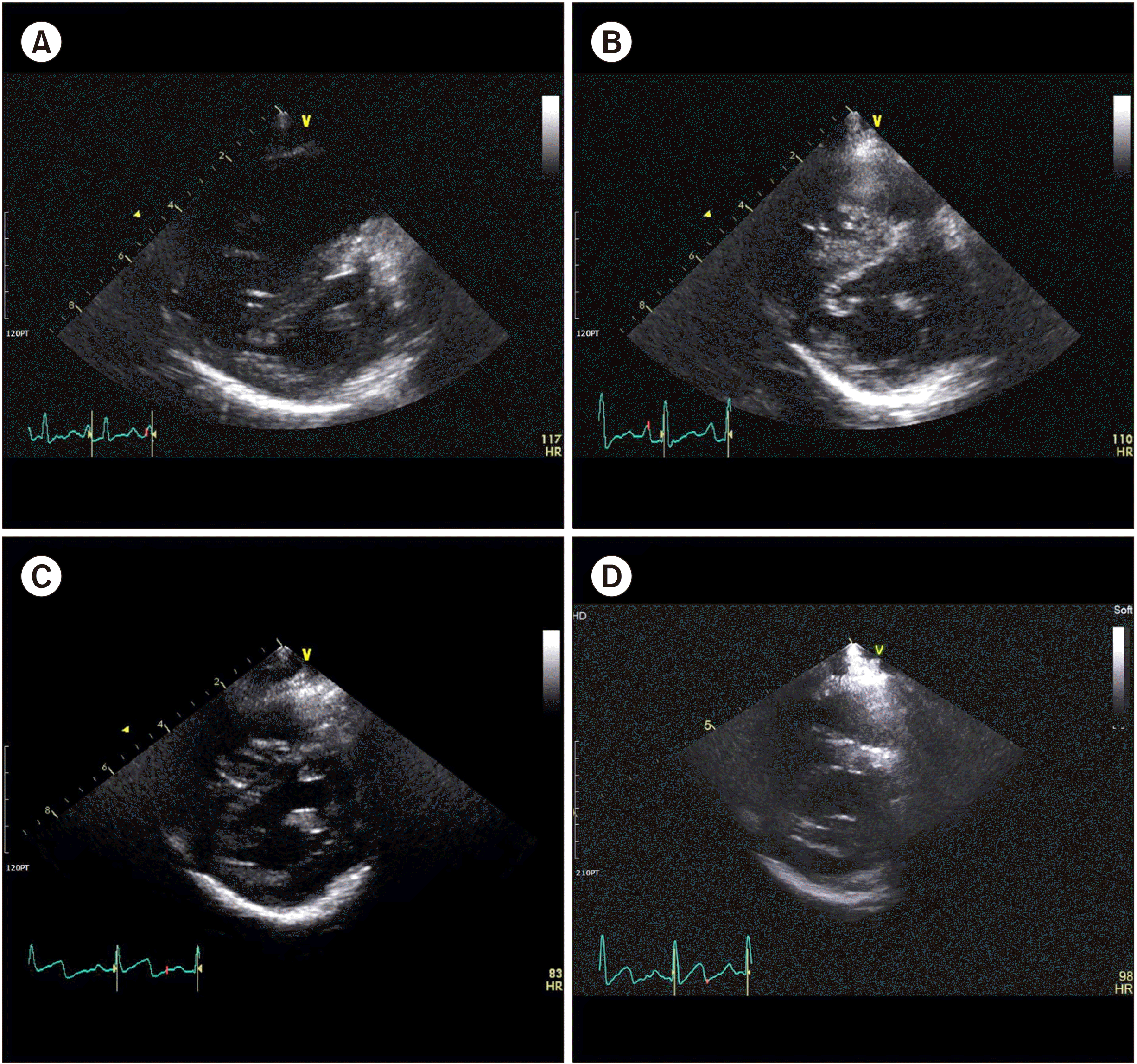
Fig. 2
Changes in the hilar-to-thoracic index with clinical course. The hilar-to-thoracic index represents the ratio of the hilar width to the transverse diameter of the thorax and correlates well with the degree of pulmonary arterial hypertension. The treatment duration of dasatinib related to the onset of PAH is presented as a charcoal-colored zone. The hilar-to-thoracic index measured on the posteroanterior chest view (CPA) is marked with diamonds (◆), and the index on the anteroposterior chest view (CAP) is marked with a cross (x). Each chest X-ray marked was obtained with full inspiration and covered almost 8 ribs. The milestones are represented by stars (*,**,***,****,*****); discharge with discontinuation of imatinib even after recovery from neutropenic fever, diagnosis of BCR-ABL1+ LL, HSCT, initiation of FK with diagnosis of skin GVHD, and diagnosis of chronic GVHD, in sequence. On reviewing a previous investigation, the change started 20 months after HSCT and became distinct 5 months later. Abbreviations: aGVHD, acute graft-versus-host disease; BCR-ABL1+ LL, BCR-ABL1+ lymphoblastic lymphoma; FK, tacrolimus; GVHD, graft-versus-host disease; HSCT, hematopoietic stem cell transplantation; MMF, mycophenolate mofetil.
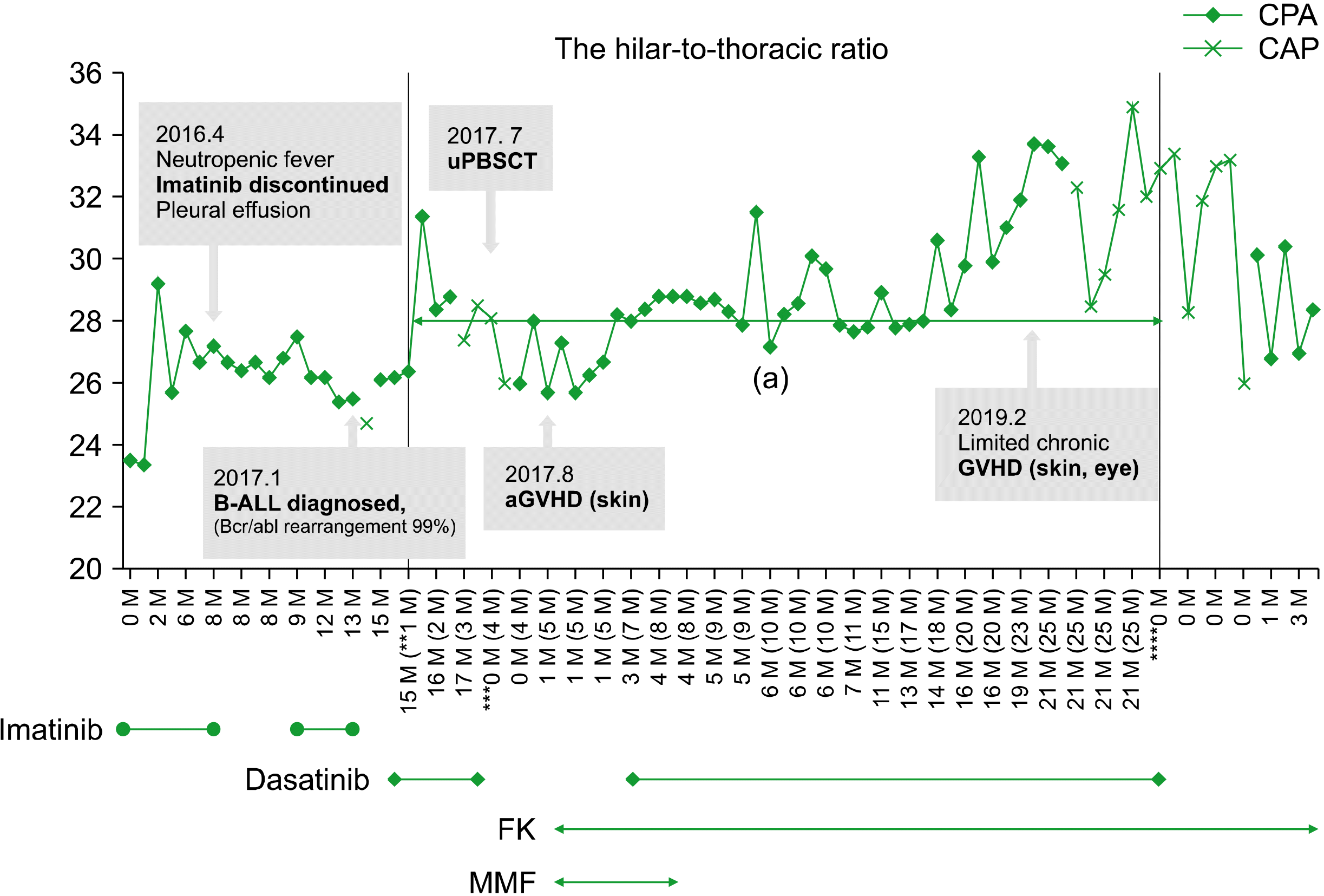
Table 1
Echocardiographic changes after discontinuation of dasatinib.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download