INTRODUCTION
Non-Hodgkin lymphoma (NHL) is a diverse group of lymphoid neoplasms categorized according to the characteristics of lymphoma cells (B, T, and natural killer cells), cell morphology, immunophenotype, genetic features, and prognosis. Children generally present with high-grade lymphomas that tend to be aggressive and fast growing, such as Burkitt lymphoma (BL), diffuse large B-cell lymphoma (DLBCL), lymphoblastic lymphoma, and anaplastic large cell lymphoma. Among them, aggressive mature B-cell lymphomas account for approximately 60% of NHLs in children [
1]. They include many subtypes and variants, such as DLBCL, BL, and high-grade B-cell lymphoma, not otherwise specified (HGBL-NOS) [
2].
Over the recent decades, the outcome of treatment with short-duration, dose-intensive, and stage-based multiagent chemotherapy in pediatric patients with mature B-cell lymphomas has considerably improved, with survival rates of >80% [
3,
4]. Since the 1980s, 3 major US and European childhood cancer groups have contributed to the most effective and proven treatment approaches to date: Lymphomes Malins B (LMB) group, Berlin–Frankfurt–Münster (BFM) group, and Children’s Cancer Group/Children’s Oncology Group (CCG/COG). The French–American–British (FAB)/LMB studies divided patients into 3 groups based on disease stage and resection status. The FAB/LMB protocols are based on the COPAD regimen (cyclophosphamide, vincristine, prednisolone, and doxorubicin). Central nervous system (CNS) prophylaxis based on high-dose (HD) methotrexate (MTX) and intrathecal injections of MTX has been proven effective [
3,
5,
6].
The BFM protocols are similar to the FAB/LMB protocols, with differences in the details of risk group stratification and treatment intensity. In BFM studies, patients were stratified into 4 risk groups according to stage, resection of localized tumors, lactate dehydrogenase (LDH) level, and CNS involvement [
7]. BFM studies revealed that patients with advanced disease benefit from more prolonged HD MTX exposure and HD cytarabine. As the LMB and BFM protocols have provided dramatic improvements in treatment outcomes in pediatric NHL, they have been the most often used treatment strategies. Recently, rituximab was added to the standard LMB chemotherapy (R-LMB), and this combination has shown efficacy in children and adolescents with high-grade, high-risk, mature B-cell NHL (B-NHL) [
8].
Since the introduction of effective intensive multiagent chemotherapy, very few children and adolescents (5–15%) with mature B-cell lymphoma have experienced refractory or relapsing disease. However, the prognosis of these patients remains poor, with survival rates of <30% [
9]. Several studies have aimed to identify risk factors to determine the prognosis of these patients. Increased serum LDH levels and CNS involvement are considered factors associated with a poor prognosis [
10]. For these groups of patients, multiple strategies have been proposed and new therapies are being investigated [
1].
The aim of our study was to investigate the clinical characteristics and treatment outcomes of children and adolescents with mature B-cell lymphoma at a single center.
Go to :

DISCUSSION
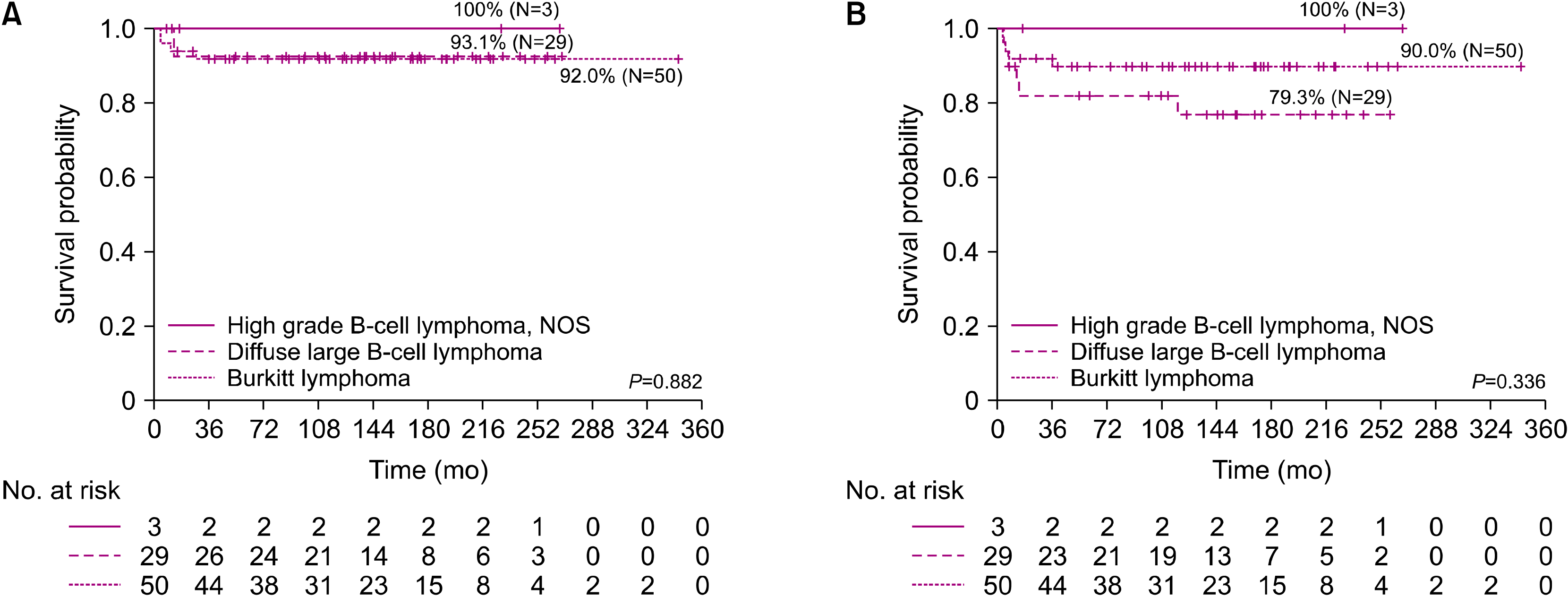
Mature B-cell lymphoma is a common type of NHL. It presents as a high-grade and aggressive tumor in the pediatric population. Several research groups have conducted various studies on chemotherapy for NHL, and the treatment strategies are complex. The prognosis of pediatric mature B-cell lymphoma has substantially improved over the decades, with good long-term survival. In 2020, the National Comprehensive Cancer Network (NCCN) guidelines for pediatric aggressive mature B-cell lymphomas provided guidance on the pathology and diagnosis, staging, initial treatment, and therapy for relapsed or refractory disease [
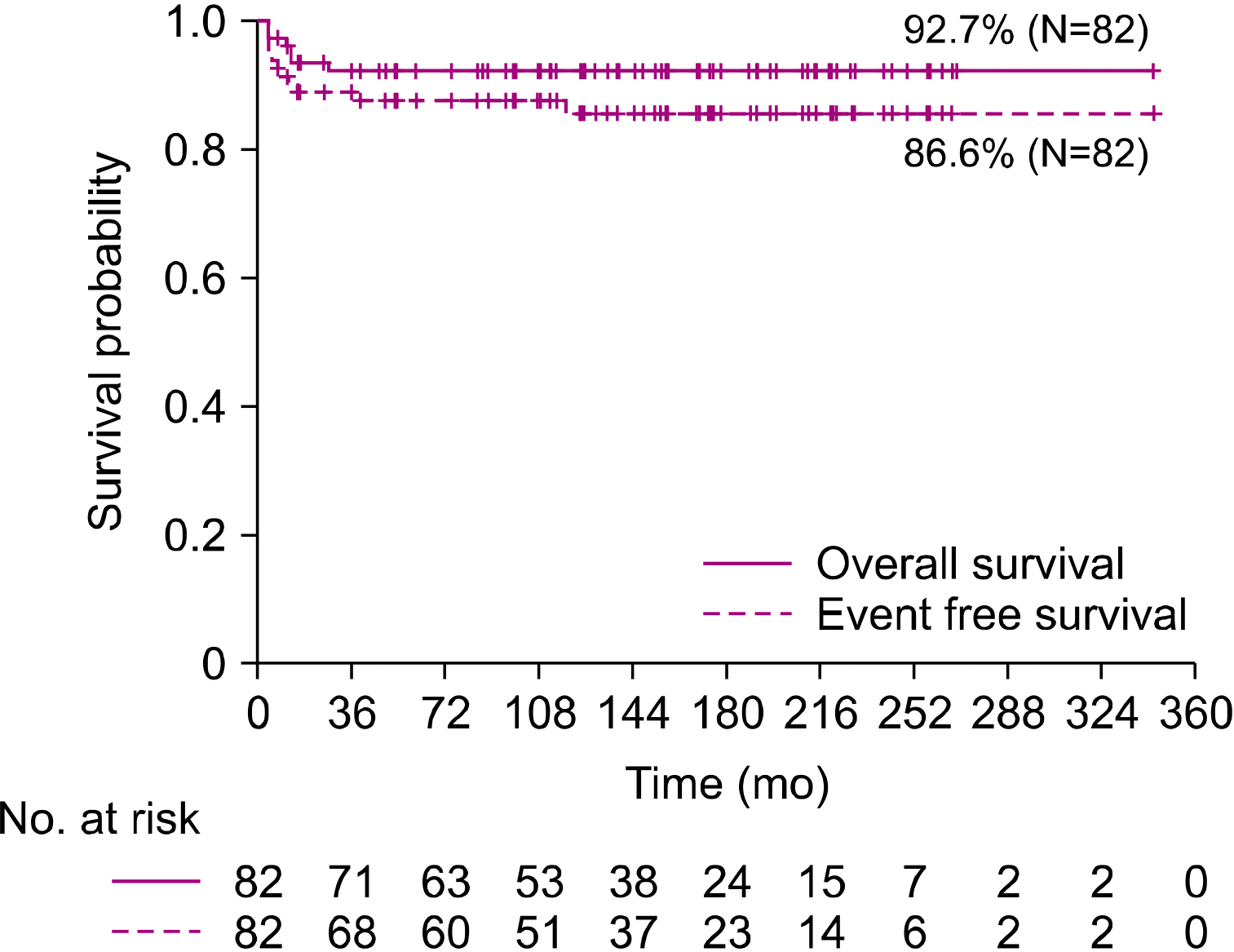
1]. Our retrospective study reviewed the clinical characteristics of patients, outcomes of various protocols, and treatments of relapsed patients with mature B-cell lymphoma over 28 years at a single institute.
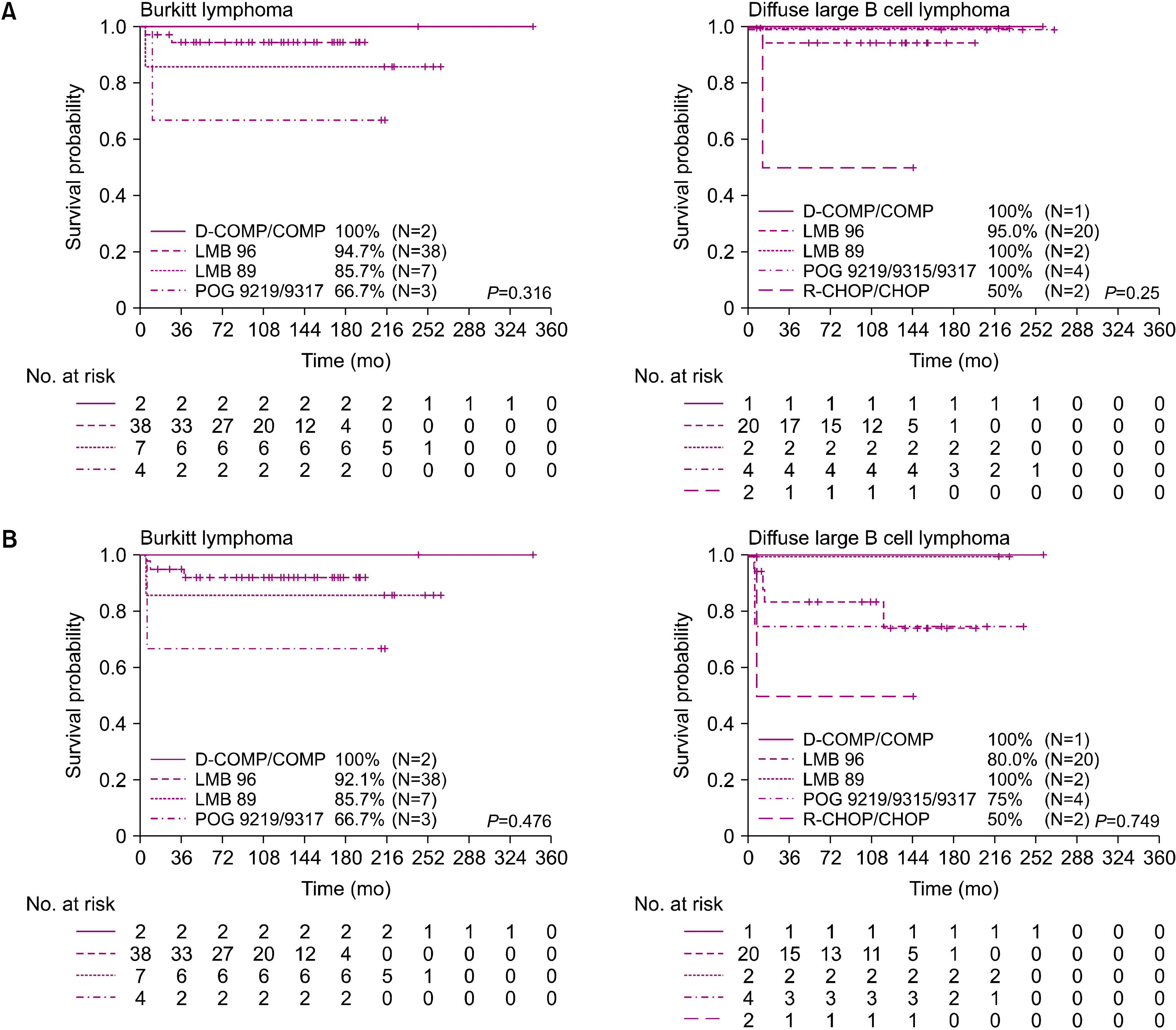
The clinical trials by the LMB, BFM, and CCG/COG groups reported EFS rates of >90% in early-stage disease and 67–79% in advanced-stage disease [
3,
6,
12,
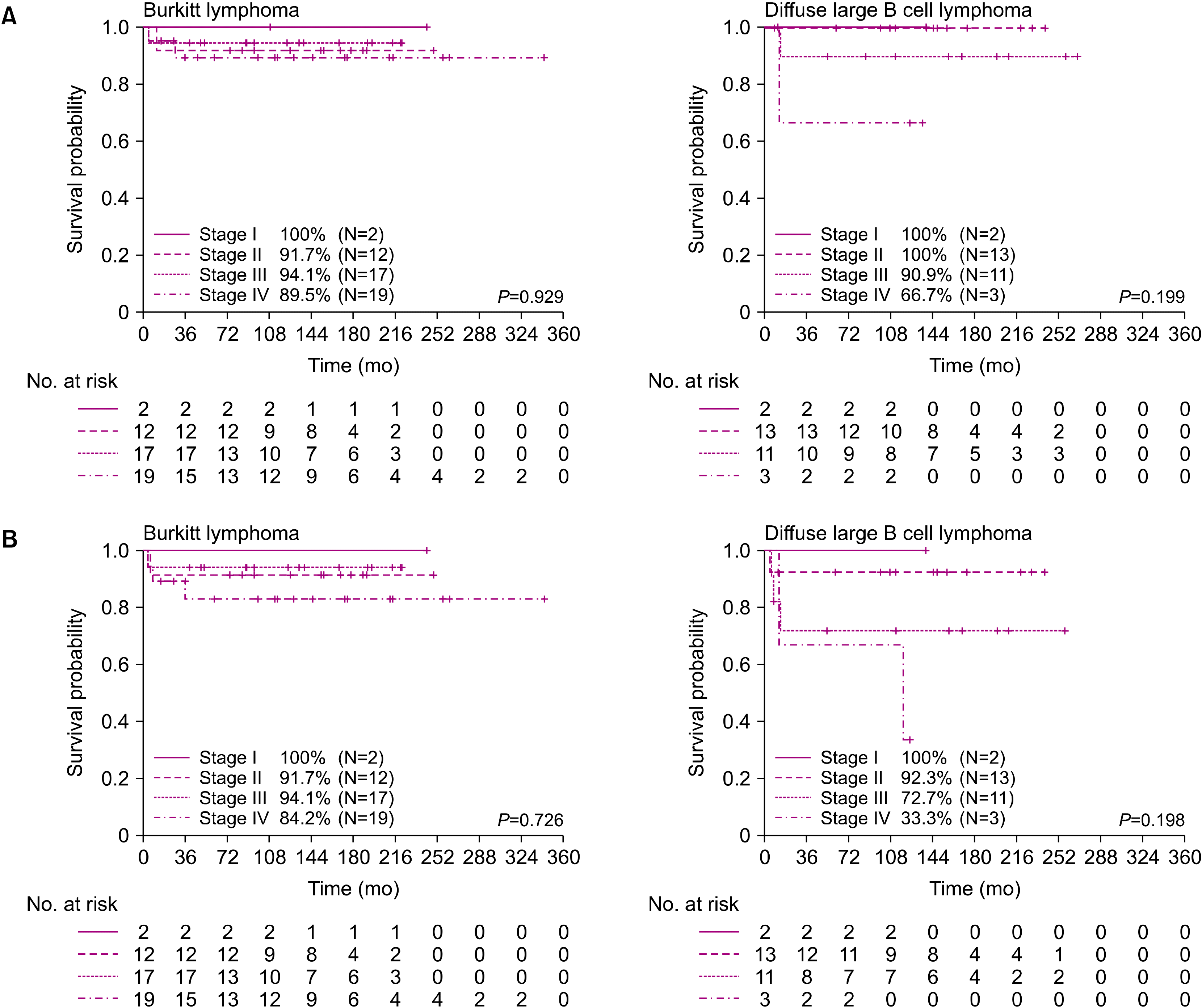
20]. In our study, the overall OS and EFS rates in our patients were 92.7% and 86.6%, respectively. Even when compared according to stage, the OS and EFS rates were 96.2% and 93.3% for stage I–II and 90.4% and 82.7% for stage III–IV, respectively. These results were comparable to those of previous studies. However, the treatment outcomes are still poor for advanced BL or DLBCL, and no standard treatment has been suggested for salvage therapy, especially in cases of relapse [
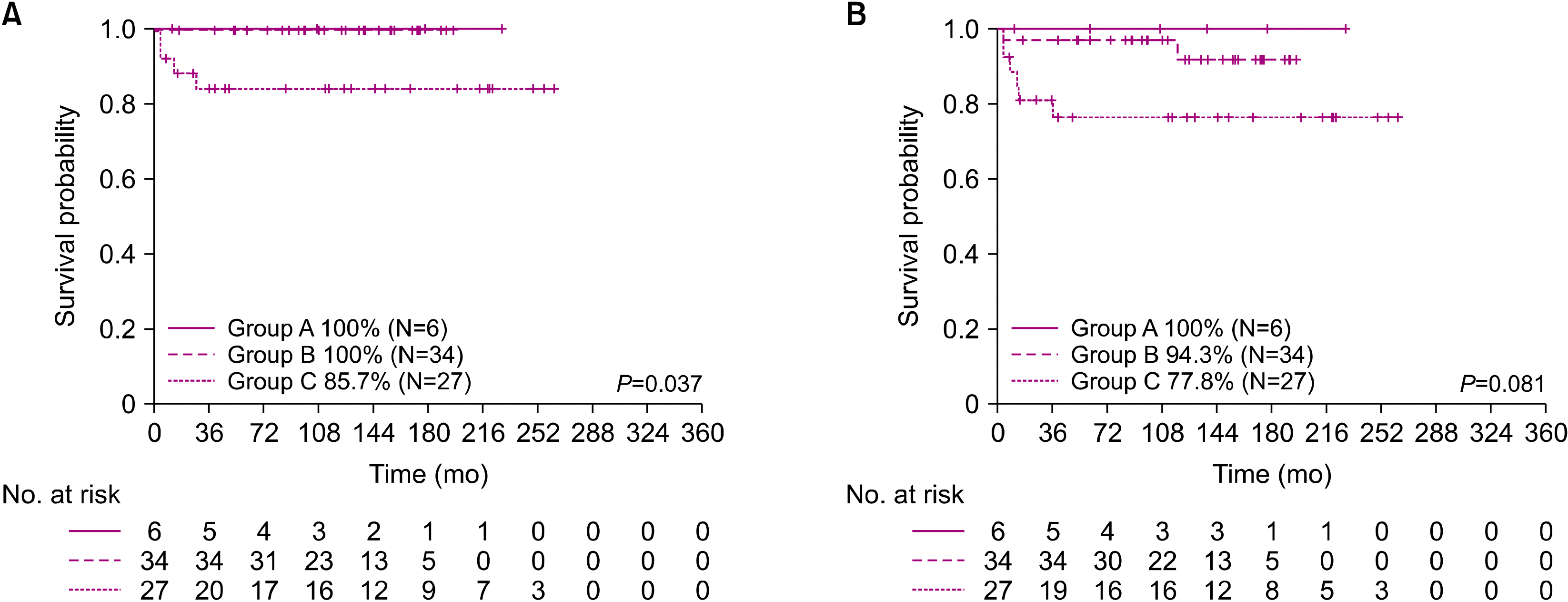
21]. In our study, stage IV DLBCL particularly had a very poor outcome even with the LMB group C protocol. The 5-year OS of patients in group C was 85.7%, with a statistically significant difference between patients in group C and those in groups A and B.
For these high-risk patients, rituximab combination therapy has been reported to provide a good outcome. Rituximab is a chimeric murine/human monoclonal antibody with a high affinity against CD20 in B cells. In adults with B-NHL, rituximab has shown efficacy and has become a standard component of care [
22]. Goldman
et al. [
19] suggested the safety and improved efficacy of therapy with the addition of rituximab compared with the standard LMB96 protocol in pediatric patients with BL who had BM (≥25% blasts) or CNS involvement. The efficacy of rituximab in high-risk patients classified as group B was also supported by Goldman
et al. [
23]. In this trial, patients with stage III/IV and group B mature B-NHL who received rituximab with the FAB/LMB96 protocol showed an improvement in the 3-year EFS rate (95% in the R-LMB group), without serious adverse events.
Recently, Minard-Colin
et al. [
8] conducted a randomized trial in 328 patients aged <18 years with high-risk (stage III with an elevated LDH level or stage IV) mature B-NHL to compare the R-LMB protocol with the standard LMB protocol alone. They reported markedly improved 3-year EFS and OS rates of >93% in the R-LMB group. On the basis of these reports, the NCCN guideline recommended rituximab-combined chemotherapy in high-risk patients classified as groups B and C [
1]. In our study, 2 patients underwent therapy with the combination of rituximab and the LMB96 protocol, both of whom showed good treatment outcomes without treatment-related complications.
In HGBL, a new separate entity with
MYC,
BCL2, or
BCL6 rearrangements, also known as double-hit or triple-hit lymphoma, has been defined in the 2016 revised WHO classification of lymphoid neoplasms [
2]. Currently, HGBL with
MYC and
BCL2 translocations accounts for approximately 10% of all lymphomas and occurs in older patients (median age, 60 yr; range, 6–82 yr) [
24]. Although several studies reported such cases as a poor prognostic group in both pediatric and adult patients [
25,
26], the recommended treatment for double-hit and triple-hit lymphomas in the pediatric population is similar to that for other high-grade mature B-cell lymphomas. Among 51 patients with available immunohistochemistry or fluorescence
in situ hybridization data in our study, 6 patients were suspicious for double-hit lymphoma with
MYC and
BCL6 rearrangements. The survival outcomes in this group could not be analyzed owing to the small number of patients; however, only 1 death occurred, in a 4-year-old patient with stage IV BL treated with the LMB96 regimen who had relapse in the CNS and BM and eventually died of progressive lymphoma. Future extensive studies on the prognosis of double-hit and triple-hit lymphomas in pediatric patients with high-grade mature B-cell lymphoma are needed.
The long-term treatment outcomes in pediatric patients with mature B-cell lymphoma have considerably improved over the recent decades. Particularly, a gradual increase in the EFS rate was achieved by eliminating local and CNS radiotherapy, reducing the total doses of cyclophosphamide and doxorubicin, and, recently, adding rituximab [
27]. Nevertheless, treatment-related toxicity and mortality remain important concerns and less toxic treatment modalities are continuously being studied. Cairo
et al. [
12] reported a toxicity-related death rate of 3%, with a high incidence of grade III/IV mucositis, infection, myelosuppression, and prolonged hospitalization during the induction courses. Tumor lysis syndrome (TLS) is also a well-known treatment-related toxicity, especially in BL, due to the high turnover rate of tumor cells. To prevent TLS, vigorous alkaline diuresis needs to be obtained, with furosemide, if necessary, and urate oxidase needs to be administered before the initiation of treatment. BL/B-cell acute lymphoblastic leukemia and LDH levels ≥500 U/L were associated with the highest incidence of TLS among patients with NHL (14.9%) [
28]. In patients treated with the LMB89 protocol, the use of urate oxidase reduced mortality due to TLS. In our study, of the total of 50 patients with BL, 15 had TLS (30.0%) and all of them had stage III/IV disease. Among the 11 patients with TLS who underwent continuous renal replacement therapy, no cases of TLS-related death occurred. Conversely, treatment-related mortality occurred in 1 male patient during consolidation chemotherapy. He died of gastrointestinal bleeding caused by myelosuppression-related thrombocytopenia.
Secondary malignancy after the treatment of pediatric NHL is also a concern for long-term treatment outcomes. In the North American Childhood Cancer Survivor Study cohort, 229 secondary malignant neoplasms (SMNs) occurred among 206 survivors treated with chemotherapy alone after a childhood cancer diagnosis [
29]. The survivors had a 1.9-fold, 4.6-fold, and 3.8-fold increased standardized incidence ratio of subsequent leukemia/lymphoma, breast cancer, and thyroid cancer, respectively. Recently, Ehrhardt
et al. [
30] reported the occurrence of SMNs in children and adolescents with NHL by investigating a total of 570 survivors over 5 years after the initial diagnosis. None of the 126 survivors treated with the contemporary LMB protocol between 1987 and 1999 developed SMNs. However, patients treated with non-LMB protocols had an SMN incidence rate of 2.2 per 1,000 person-years (5,871 total person-years). Two cases of SMN were observed in our study: therapy-related acute myeloid leukemia and thyroid cancer. These were successfully treated with allogeneic HSCT and surgery.
Our study had several limitations. First, it was a retrospective study with a small sample of patients recruited from a single center. Second, as the criteria for selecting the treatment strategy have changed over the last 25 years, various treatment regimens were applied with different indications. Therefore, the number of patients significantly differed among the different treatment protocols, making it difficult to compare the treatment outcomes. In addition, detailed data on treatment-related toxicities for each chemotherapy regimen were not available.
In summary, this study revealed favorable treatment outcomes in children and adolescents with aggressive mature B-cell lymphoma despite the long study period (>25 yr). However, further studies are needed to improve the survival rate of patients with advanced-stage disease or disease relapse. Targeted therapies such as rituximab are expected to become more widely used to improve the outcomes of high-risk patients. In addition to efforts to improve the survival rates, careful monitoring for acute toxicity or secondary malignancy due to intensive multidrug chemotherapy is required. The development of guidelines for the follow-up observation of these patients may help in monitoring chemotherapy-related complications.
Go to :





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download