Abstract
Background
Antifungal prophylaxis is recommended for hematopoietic stem cell transplantation (HSCT) to decrease the incidence of invasive fungal infections (IFI). This study aimed to compare the two groups of antifungal prophylaxis in pediatric patients undergoing allogeneic HSCT.
Methods
This observational, analytic, retrospective cohort study compared the incidence of IFI with antifungal prophylaxis with voriconazole vs. other antifungals in the first 100 days after allogeneic HSCT in patients aged <18 years between 2012 and 2018. The statistical analysis included univariate and multivariate analyses and determination of the cumulative incidence of invasive fungal infection by the Kaplan‒Meier method using STATA 14 statistical software.
Results
A total of 139 allogeneic HSCT were performed. The principal diagnosis was acute leukemia (63%). The 75% had haploidentical donors, and 50% used an antifungal in the month before transplantation. Voriconazole (69%) was the most frequently administered antifungal prophylaxis. The cumulative incidence of IFI was 5% (7 cases). Of the patients with IFIs, four began prophylaxis with voriconazole, one with caspofungin, and one with fluconazole. Additionally, six were possible cases, one was proven (Candida parapsilosis), and 1/7 died.
Invasive fungal infections (IFIs) are opportunistic pathologies with potentially preventable serious complications, mainly in immunocompromised patients, with high morbidity and mortality rates [1].
Risk factors for the development of IFI include host factors, factors related to the underlying disease, factors associated with treatment, complications [2] and in some cases, environmental factors [3-5].
The overall IFI incidence is 2–16%, depending on transplant characteristics, diagnostic methods, and follow-up [6-8]. The most common etiological agents are Candida spp. and Aspergillus spp. [6], with 60–95% mortality rate in transplant patients [7].
Antifungal prophylaxis has reduced the number of fungal infections in patients who have undergone allogeneic hematopoietic stem cell transplantation (AHSCT) [9]. Different regimens are used in pediatric patients, including fluconazole [10], voriconazole [11, 12], itraconazole [13], caspofungin [14], and posaconazole [15]. Voriconazole has shown an acceptable tolerance profile, manageable and transient adverse events, and adequate antifungal coverage against filamentous fungi [16]. Although prophylaxis has decreased the number of IFI cases, this pathology continues to be a relevant complication in immunocompromised patients [17].
This study aimed to compare the outcome variables of pediatric patients who underwent AHSCT and received antifungal prophylaxis, such as voriconazole with those who received other antifungals at Fundación Valle del Lili, Cali, Colombia, from 2012 to 2018.
This was an observational, analytical, retrospective cohort study of pediatric patients with AHSCT between 2012 and 2018. Data were collected from the electronic medical records of all patients aged <18 years who underwent AHSCT at the Fundación Valle del Lili. The exclusion criteria were patients with previous IFI. The Ethics Committee on Biomedical Research approved this study under code 1442.
According to the literature, IFIs are proven probable and possible [18]. Proven when it met a patient criterion (bone marrow transplant, neutropenia, prolonged use of corticosteroids or immunosuppressants or carrier of severe congenital immunodeficiency), plus a clinical criterion (tracheo-bronchitis, sinusitis, central nervous system infection, disseminated candidiasis or lower respiratory tract fungal infection), and a microbiological criterion (identification of the agent by microscopic analysis of sterile biopsy or aspirate material, culture of sterile material or blood). Probable when there was a host criterion plus a clinical criterion and microbiological criterion (direct or indirect tests that found fungal elements). Possible if it meets the patient criterion plus at least one clinical diagnosis without microbiological support.
Antifungal prophylaxis: The antifungal agent used between day 0 and day +100 was considered until the IFI development or death. The regimen included intravenous voriconazole at a dose of 8 mg/kg twice a day without a loading dose for children between 2 and 11 years old and adolescents aged 12–14 years with <50 kg body weight, or 4 mg/kg twice a day for adolescents aged 12 to 14 years with ≥50 kg body weight and all adolescents over 14 years [19, 20]. Caspofungin was administered at a dose of 50 mg/m2/day and fluconazole at a dose of 5–7 mg/kg/day. Posaconazole (300 mg/day) Liposomal amphotericin B was administered at a prophylactic dose of 1 mg/kg/day [21]. According to the pharmaceutical recommendation, itraconazole was administered at a starting dose of 5 mg/kg on days 1 and 2 and a continuation dose of 2.5 mg/day. In any adverse event or complication, the drug related to the event should be changed.
The conditioning regimen was classified as myeloablative or reduced in intensity [22]. Graft-versus-host disease (GVHD) prophylaxis was performed according to the transplant type [23-25]. The graft was defined according to the literature [26]. The Glucksberg criteria have been used to grade acute GVHD [27]. The cause of death within +100 days post-AHSCT was defined according to literature [28].
A comparative statistical analysis of the incidence of IFI between patients who received initial prophylaxis with voriconazole and those who received other antifungals was performed (total group, N=139). All variables were evaluated for up to 100 days after AHSCT. The comparison groups were selected considering that voriconazole is the most widely used prophylactic antifungal drug in the institution, and other antifungals constituted a smaller proportion.
Continuous variables are expressed as medians and interquartile ranges, and comparisons were performed using the Mann–Whitney test. Categorical variables are expressed as proportions and compared using the chi-square or Fisher’s exact test, as appropriate. Statistical significance was defined as P<0.05. A multivariate analysis was performed with logistic regression that included risk factors with P-values <0.2 and other known factors for IFI, regardless of P-value, using the stepwise backward methodology to select the variables in the final model. Subgroup analysis comparing voriconazole and fluconazole was performed (N=119). Survival analysis and cumulative incidence estimation of IFI were performed using the Kaplan–Meier method. The log-rank test was used to compare the cumulative incidence of IFI between the groups. The statistical software STATA 14 was used for the data analysis.
A total of 139 patients were evaluated and divided into two groups according to the initial antifungal dose. Of these, 96 were administered with voriconazole, and 43 had other antifungals, including fluconazole [23], caspofungin [14], posaconazole [4], and amphotericin B [4]. The median age was 9.7 years (IQR, 4.7–13), and 41% of the patients were women. The most frequent pathology was acute leukemia (63%). The donors were haploidentical (75%), matched (23%), and cord (2%). The stem cell sources were bone marrow (65%), peripheral blood (29.5%), combined bone marrow and peripheral blood (3.5%), and umbilical cord blood (2%). Conditioning was myeloablative in 55% of patients, and 34% used ATG during conditioning.
Prophylaxis for acute GVHD was post-transplant cyclophosphamide-based in 78% and cyclosporine-based in 23% (Table 1). The median neutrophil and platelet graft times were 16 days. In the month before AHSCT, 50% of patients received one or more antifungals, mainly fluconazole and voriconazole. The 48% received one or more antimicrobial therapies, and 24% received steroids (Tables 1, 2). Seven patients had IFI (one proven, and six possible).
The 100-day cumulative incidence of IFI was 5%. Seven patients had an IFI. One was proven by bronchoalveolar lavage culture (one Candida parapsilosis), and six were possible. Four patients received prophylactic voriconazole, and three received another antifungal agent. In this group, one (11%) died of multiple organ failure at 15 days (Table 3).
Acute GVHD grades III–IV occurred in 11% of all patients, and moderate-to-severe mucositis occurred in 43%. Fifty-one percent had other infections, 7% of which were catheter-associated. Primary graft failure occurred in 4.5% of those one with neutrophil and platelet graft failure and six with platelet graft failure. Twenty-four (17%) patients died, of which seven died before 28 days. Of all deaths, three (12.5%) were due to the disease, and 21 (87.5%) were due to AHSCT-related causes (Table 4). The 100-days overall survival (OS) rate was 81% (Fig. 1).
Some of the clinical characteristics of the study showed differences between those who received voriconazole and those who received other antifungal prophylaxis. To control for the differences between these groups, we performed multivariate analysis for IFI diagnosis. We included the following variables: voriconazole prophylaxis, moderate-to-severe mucositis, malignant-based disease, acute GVHD grade III–IV, and myeloablative conditioning regimen. However, there were no statistically significant differences (Table 5); therefore, we performed a subgroup analysis (Table 6). Acute GVHD grade III–IV is a risk factor for IFI, independent of the other variables analyzed.
In this single-centered retrospective cohort study, two antifungal prophylaxis regimens (voriconazole or other antifungals) were used from the time of transplantation to 100 days after AHSCT, compared to 139 pediatric patients treated from 2012 to 2018 at a reference center in Cali, Colombia. There were seven cases of post-AHSCT IFI (one proven and six possible). No differences were found in the incidence of IFI between the two prophylaxis groups.
In this study, the 100-day cumulative incidence of IFI was 5%, similar to that reported by Hazar et al. [6], who reported an incidence of 5% after excluding cases of possible IFI. However, our results differed from those with Hovi et al. [7], who reported an incidence of 12%, which is higher than our results.
The pathogen isolated in proven IFI was Candida parapsilosis, similar to that reported in other studies [6-8, 29]. Of the seven patients with IFI, only one (11%) died of multiorgan failure, without direct association with IFI. Our incidence of mortality contrasts with the deaths due to IFI reported in the literature between 20–70% [29, 30]. This may be due to the small number of IFI cases in the study population.
The use of an antifungal prophylaxis protocol in transplant patients helps protect them against infection during the period of immunosuppression and neutropenia [17], which typically includes the first +100 days. Therefore, individualized antifungal prophylaxis based on patient characteristics and local epidemiology is essential to identify the groups most at risk of infection and to provide the best option for each patient. Voriconazole is the most widely used prophylactic antifungal agent (69% of patients) because of its broad-spectrum activity, especially against filamentous fungi such as Aspergillus, which distinguishes it from fluconazole [11].
This study has some limitations. First, this was a retrospective design and data collection from electronic medical records could have lacked relevant data. Second, this had a short follow-up period, which could have affected the study results. Third, it was conducted only in a single institution in the country. However, this study revealed important information about IFI cases occurring in a transplant unit in our population.
In conclusion, we found no differences in the incidence of IFI between patients who received antifungal prophylaxis with voriconazole or other antifungal medications. Studies with larger sample sizes are required to identify independent risk factors for post-transplant IFI. In the Azoles group, it was observed that the presence of GVHD III–IV was an independent risk factor for IFI in the multivariate analysis performed for the subgroup.
REFERENCES
1. Badiee P, Hashemizadeh Z. 2014; Opportunistic invasive fungal infections: diagnosis & clinical management. Indian J Med Res. 139:195–204. PMID: 24718393. PMCID: PMC4001330.
2. Ramos JT, Francisco L, Daoud Z. 2016; Invasive fungal infections in children: similarities and differences with adults. Rev Esp Quimioter. 29(Suppl 1):59–65. PMID: 27608317.
3. Cruz-Contreras D. 2016; Aspergilosis invasiva en el paciente que recibe trasplante alogénico de células progenitoras hematopoyéticas: epidemiología , diagnóstico, profilaxis y tratamiento. Rev Hematol Mex. 17:262–7.
4. Omrani AS, Almaghrabi RS. 2017; Complications of hematopoietic stem transplantation: fungal infections. Hematol Oncol Stem Cell Ther. 10:239–44. DOI: 10.1016/j.hemonc.2017.05.013. PMID: 28636889.

5. Schwartz KL, Sheffield H, Richardson SE, Sung L, Morris SK. 2015; Invasive fusariosis: a single pediatric center 15-year experience. J Pediatric Infect Dis Soc. 4:163–70. DOI: 10.1093/jpids/pit080. PMID: 26407418.

6. Hazar V, Karasu GT, Uygun V, et al. 2019; Risks and outcomes of invasive fungal infections in pediatric allogeneic hematopoietic stem cell transplant recipients receiving fluconazole prophylaxis: a multicenter cohort study by the Turkish Pediatric Bone Marrow Transplantation Study Group. Med Mycol. 57:161–70. DOI: 10.1093/mmy/myy015. PMID: 29608706.

7. Hovi L, Saarinen-Pihkala UM, Vettenranta K, Saxen H. 2000; Invasive fungal infections in pediatric bone marrow transplant recipients: single center experience of 10 years. Bone Marrow Transplant. 26:999–1004. DOI: 10.1038/sj.bmt.1702654. PMID: 11100280.

8. Czyżewski K, Styczyński J, Giebel S, et al. 2019; Age-dependent determinants of infectious complications profile in children and adults after hematopoietic cell transplantation: lesson from the nationwide study. Ann Hematol. 98:2197–211. DOI: 10.1007/s00277-019-03755-2. PMID: 31321454. PMCID: PMC6700048.

9. Choi JK, Cho SY, Yoon SS, et al. 2017; Epidemiology and risk factors for invasive fungal diseases among allogeneic hematopoietic stem cell transplant recipients in Korea: results of "RISK" Study. Biol Blood Marrow Transplant. 23:1773–9. DOI: 10.1016/j.bbmt.2017.06.012. PMID: 28668492.

10. Ullmann AJ, Lipton JH, Vesole DH, et al. 2007; Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med. 356:335–47. DOI: 10.1056/NEJMoa061098. PMID: 17251530.

11. Sano H, Kobayashi R, Hori D, et al. 2018; Prophylactic administration of voriconazole with two different doses for invasive fungal infection in children and adolescents with acute myeloid leukemia. J Microbiol Immunol Infect. 51:260–6. DOI: 10.1016/j.jmii.2016.05.002. PMID: 27329132.

12. Molina JR, Serrano J, Sánchez-García J, et al. 2012; Voriconazole as primary antifungal prophylaxis in children undergoing allo-SCT. Bone Marrow Transplant. 47:562–7. DOI: 10.1038/bmt.2011.111. PMID: 21572466.

13. Marr KA, Crippa F, Leisenring W, et al. 2004; Itraconazole versus fluconazole for prevention of fungal infections in patients receiving allogeneic stem cell transplants. Blood. 103:1527–33. DOI: 10.1182/blood-2003-08-2644. PMID: 14525770.

14. Mariotti J, De Philippis C, Bramanti S, et al. 2019; Caspofungin for primary antifungal prophylaxis after T-cell-replete haploidentical stem cell transplantation with post-transplant cyclophosphamide. Eur J Haematol. 102:357–67. DOI: 10.1111/ejh.13214. PMID: 30672611. PMCID: PMC7163667.

15. Rosanova MT, Voto C, Mussini MS, et al. 2018; Uso de posaconazol en niños: experiencia en un hospital pediátrico de alta complejidad. Arch Argent Pediatr. 116:e451–4. DOI: 10.5546/aap.2018.e451.

16. Pana ZD, Kourti M, Vikelouda K, et al. 2018; Voriconazole antifungal prophylaxis in children with malignancies: a nationwide study. J Pediatr Hematol Oncol. 40:22–6. DOI: 10.1097/MPH.0000000000000926. PMID: 28816795.

17. Omer AK, Ziakas PD, Anagnostou T, et al. 2013; Risk factors for invasive fungal disease after allogeneic hematopoietic stem cell trans-plantation: a single center experience. Biol Blood Marrow Transplant. 19:1190–6. DOI: 10.1016/j.bbmt.2013.05.018. PMID: 23747459.

18. De Pauw B, Walsh TJ, Donnelly JP, et al. 2008; Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) C. Clin Infect Dis. 46:1813–21. DOI: 10.1086/588660. PMID: 18462102. PMCID: PMC2671227.

19. L Kandaurava S, S Baslyk K, A Migas A, et al. 2020; Comparative study of prophylaxis with high and low doses of Voriconazole in children with malignancy. Curr Med Mycol. 6:27–34. DOI: 10.18502/cmm.6.4.5331. PMID: 34195457. PMCID: PMC8226053. PMID: 9f572bc1f306441288b05208de240928.
20. Asociación Española de Pediatría. 2021. Voriconazol. Pediamecum. Asociación Española de Pediatría;Madrid, Spain: at https://www.aeped.es/pediamecum/generatepdf/api?n=83577. Accessed January 5, 2021.
21. Mendoza-Palomar N, Soques E, Benitez-Carabante MI, et al. 2020; Low-dose liposomal amphotericin B for antifungal prophylaxis in paediatric allogeneic haematopoietic stem cell transplantation. J Antimicrob Chemother. 75:2264–71. DOI: 10.1093/jac/dkaa149. PMID: 32335674.

22. Bacigalupo A, Ballen K, Rizzo D, et al. 2009; Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 15:1628–33. DOI: 10.1016/j.bbmt.2009.07.004. PMID: 19896087. PMCID: PMC2861656.

23. Kanda Y, Hyo R, Yamashita T, et al. 2006; Effect of blood cyclosporine concentration on the outcome of hematopoietic stem cell transplantation from an HLA-matched sibling donor. Am J Hematol. 81:838–44. DOI: 10.1002/ajh.20710. PMID: 16888784.

24. Al-Homsi AS, Roy TS, Cole K, Feng Y, Duffner U. 2015; Post-transplant high-dose cyclophosphamide for the prevention of graft-versus-host disease. Biol Blood Marrow Transplant. 21:604–11. DOI: 10.1016/j.bbmt.2014.08.014. PMID: 25240817.

25. Medina D, Estacio M, Rosales M, Manzi E. 2020; Haploidentical stem cell transplant with post-transplantation cyclophosphamide and mini-dose methotrexate in children. Hematol Oncol Stem Cell Ther. 13:208–13. DOI: 10.1016/j.hemonc.2020.01.003. PMID: 32224144.

26. Holler E, Greinix H, Zeiser R. 2019; Acute graft-versus-host disease. In: Carreras E, Dufour C, Mohty M, Kröger N, eds. The EBMT handbook: hematopoietic stem cell transplantation and cellular therapies. 7th ed. Cham, Switzerland:. Springer,. 323–30. DOI: 10.1007/978-3-030-02278-5_43. PMID: 32091805.
27. Rowlings PA, Przepiorka D, Klein JP, et al. 1997; IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br J Haematol. 97:855–64. DOI: 10.1046/j.1365-2141.1997.1112925.x. PMID: 9217189.

28. Hahn T, Sucheston-Campbell LE, Preus L, et al. 2015; Establishment of definitions and review process for consistent adjudication of cause-specific mortality after allogeneic unrelated-donor hemato-poietic cell transplantation. Biol Blood Marrow Transplant. 21:1679–86. DOI: 10.1016/j.bbmt.2015.05.019. PMID: 26028504. PMCID: PMC4537799.

29. Carlesse F, Daudt LE, Seber A, et al. 2019; A consensus document for the clinical management of invasive fungal diseases in pediatric patients with hematologic cancer and/or undergoing hematopoietic stem cell transplantation in Brazilian medical centers. Braz J Infect Dis. 23:395–409. DOI: 10.1016/j.bjid.2019.09.005. PMID: 31738887.

30. Panichella M, Epelbaum C, Rosanova MT, et al. 2016; Infecciones fúngicas en pacientes hemato-oncológicos pediátricos/fungal infections in pediatric hematology-oncology patients. Med Infant. 23:18–23.
Fig. 1
100-days overall survival was 81% (A), the 100-day cumulative incidence of invasive fungal infection was 5.3% (B), and incidence of invasive fungal infection according to prophylaxis with voriconazole (4.4%) or another (7.4%) antifungal (C) at 100 days post-allogeneic hematopoietic stem cell trans-plantation.
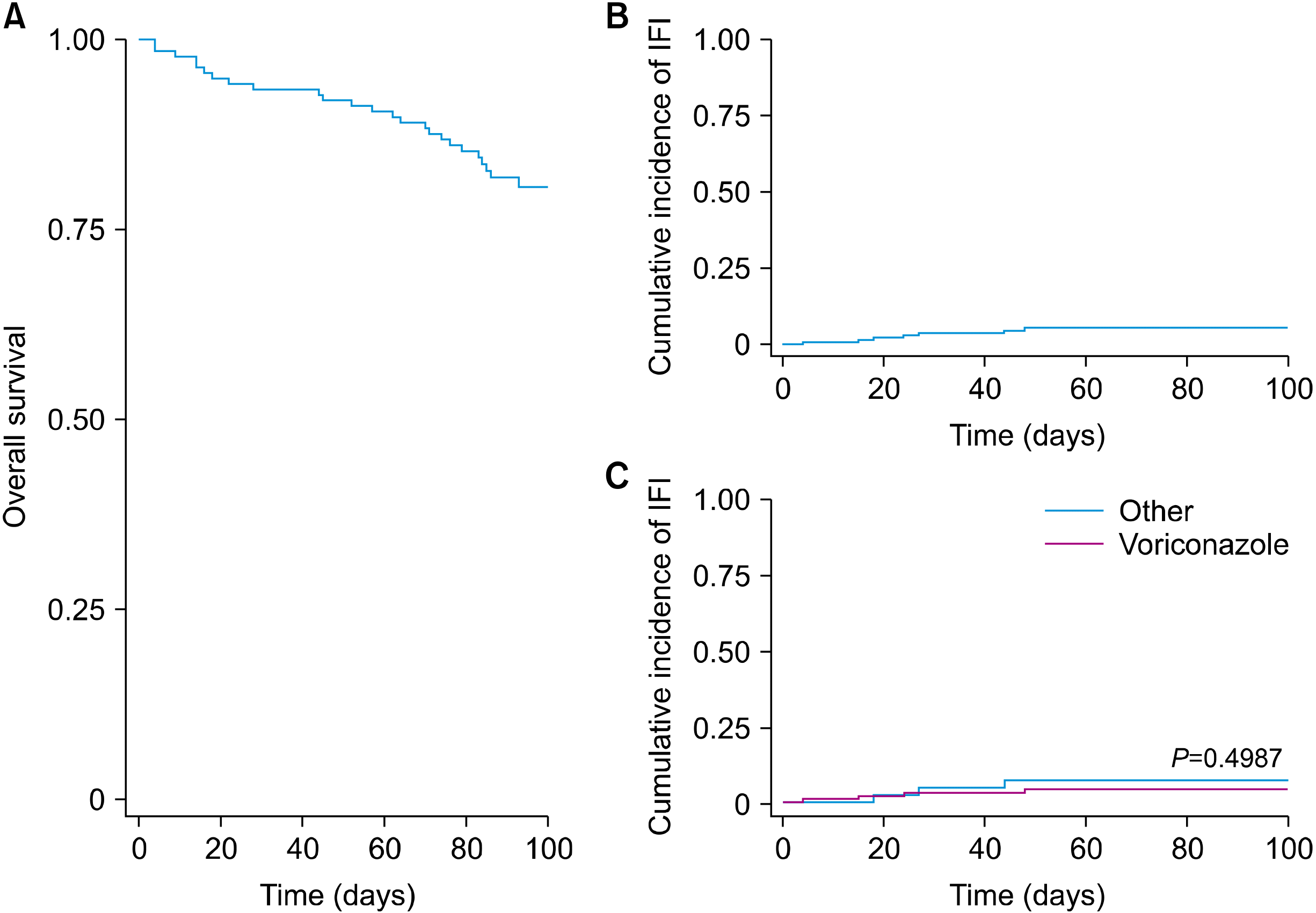
Table 1
Demographic and clinical characteristics.
Table 2
Previous antifungal/antimicrobial therapies and IFI cases.
Table 3
Characteristics of patients with IFI.
Table 4
Posttransplant outcomes and complications.
| Variable | Overall (N=139) | Voriconazole (N=96) | Other (N=43) | P |
|---|---|---|---|---|
| Acute GVHD grade III-Iva), N (%) | 15 (11) | 9 (10) | 6 (15) | 0.440 |
| ARDS, N (%) | 11 (8) | 10 (10.4) | 1 (2.3) | 0.102 |
| Hemorrhagic cystitis, N (%) | 39 (28) | 30 (31) | 9 (21) | 0.211 |
| Veno-occlusive disease, N (%) | 8 (6) | 6 (6) | 2 (4.6) | 0.708 |
| Moderate to severe mucositis, N (%) | 60 (43) | 45 (47) | 15 (35) | 0.187 |
| Infection, N (%) | 71 (51) | 53 (55) | 18 (42) | 0.146 |
| Catheter infection, N (%) | 5 (7) | 5 (9) | 0 (0) | 0.177 |
| Primary graft failureb), N (%) | 6 (4.5) | 5 (5.5) | 1 (2.4) | 0.415 |
| Deaths, N (%) | 24 (17) | 19 (20) | 5 (12) | 0.239 |
| Disease-related, N | 3/24 | 2/19 | 1/5 | |
| Transplant related, N | 21/24 | 17/19 | 4/5 | |
| GVHD | 3 | 2 | 1 | |
| Bacterial infections, N | 4 | 4 | 0 | |
| P. aeruginosa, N | (2) | (2) | 0 | |
| K. pneumoniae, N | (1) | (1) | 0 | |
| S. epidermidis, N | (1) | (1) | 0 | |
| Viral infections | 5 | 3 | 2 | |
| Adenovirus | (2) | (1) | (1) | |
| CMV | (3) | (2) | (1) | |
| Bleeding | 1 | 1 | 0 | |
| Multiple organ | 1 | 1 | 0 | |
| Failure, N | 8 | 7 | 1 |
Table 5
Risk factors for the development of IFI in the total group: a univariate and multivariate analysis.
Table 6
Risk factors for the development of IFI in the subgroup: a univariate and multivariate analysis.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download