Abstract
Background
Acute lymphoblastic leukemia (ALL) is a malignant clonal bone marrow disorder with a high mortality rate during the initial therapy. This retrospective study aimed to describe and analyze the risk factors and causes of induction-related mortality (IRM) in an adolescent and adult ALL population treated in a low- and middle-income country.
Methods
From 2009 to 2016, a total of 167 patients were included, of which 50.9% were male with a median age of 28 years. B-immunophenotype represented 97.6%, and high-risk cytogenetics were present in 23.3%. During induction therapy, 91% had at least 1 complication, most of which were infectious, with an IRM of 12%.
Acute lymphoblastic leukemia (ALL) is characterized by a malignant transformation and subsequent clonal expansion of lymphoid progenitors, associated with various genetic and molecular abnormalities [1]. Diagnostic and therapeutic advances over the last decades have enabled high-income countries to achieve first complete remission (CR1) rates of >80%, improvements in overall survival (OS), and a decreased treatment- and induction-related mortality (IRM) [2]. Conversely, compared with the <5% IRM reported in these settings, in low- and middle-income countries (LMICs), such as Mexico and Brazil, it remains as high as 17% to 26% [3-5].
Differences in clinical outcomes between adult and pediatric populations have been attributed to different first-line therapeutic regimens and to the favorable disease biology amongst children [6]. Adolescents and young adults (AYAs) are a particular subgroup facing important management challenges due to their transitioning position and increased likelihood of presenting with high-risk ALL features. Compared to that of children, AYAs have a greater incidence of Philadelphia chromosome positivity (Ph+), Philadelphia-like, and BCL2/MYC rearrangements, and decreased frequency of hyperdiploidy and ETV6/RUNX1-positivity [7]. Reports on long-term clinical outcomes have shown a decreased 5-year OS with increased mortality in CR1 for AYAs, mostly due to severe infectious diseases and IRM [8].
The following are known risk factors for IRM: male sex, T-cell ALL, high risk ALL, low platelet count and white blood cell count >100,000 at diagnosis, and patients with longer travel time to the clinic [9, 10]. However, all this information comes exclusively from pediatric cohorts. To address the paucity of literature on AYAs and adults in LMICs, this study aimed to determine the cohort rates, causes, and risk factors of IRM.
We conducted a retrospective study including consecutive patients aged ≥16 years with newly diagnosed ALL at our institution from January 2009 to September 2016. Patients with incomplete clinical records, those that received induction therapy prior to referral to our center, and those lost to follow-up before day 60 after induction were excluded. The study was approved by our center´s Institutional Review Board (HEM-3679-21-22-1).
Clinical, laboratory, and outcomes data were collected from the physical and/or electronic medical records. Patients were classified as AYAs if their age was ≤40 years. Socioeconomic status (SES) was defined according to monthly household income as follows: low-SES, <180.00 USD; middle-SES, ≥180.00 USD; and high-SES, patients with private health insurance coverage. Performance status (PS) was stratified according to the Eastern Cooperative Oncology Group (ECOG) Performance Scale [11], and records were obtained at the time of ALL diagnosis.
ALL diagnosis was made in accordance with the 2016 WHO Classification of Tumors of Hematopoietic and Lymphoid Tissues [12]. A high-risk cytogenetic profile was defined as the presence of the following: translocation t(9,22) (Ph+ positive), mixed-lineage leukemia 1 (MLL1) rearrangements, hypodiploidy, and/or complex karyotypes. Central nervous system (CNS) status was defined as CNS-1, -2, or -3 according to the NCCN Guidelines [13]. Based on previously published protocols, treatment regimens were grouped into Hyper-VAD [14], our institutional protocol (HOP 0195/0612) [15], and other regimens.
IRM was defined as death within 60 days after the initiation of the induction regimen, during post-chemotherapy myelosuppression period, and not being associated with disease refractoriness or progression. Cause of death was defined as infectious if it was directly associated with septic shock or other complications related to an infectious disease, hematologic if it was attributed to major hemorrhage or thrombotic events, or other when it was none of the above. Overall survival (OS) was defined in days, considering the time from diagnosis to death or last follow-up.
Complications were reported as infectious, metabolic, or hematologic. Infectious complications included bloodstream, urinary tract, and skin and soft tissue infections, as well as pneumonia, invasive fungal infections, and C. difficile colitis. Metabolic complications comprised hipertransaminasemia, renal impairment with or without dialysis requirement, and tumor lysis syndrome. Major hemorrhagic or thrombotic events and disseminated intravascular coagulation (DIC) were considered hematologic complications. All complications included in the variables were those that developed between day 1 and 60 of induction treatment.
Qualitative data were described in terms of frequencies and percentages, and quantitative variables were described in terms of median and range. Mortality curves were constructed via the Kaplan-Meier method, and univariate survival analysis was performed using the log-rank test. Cox regression was performed for multivariable analysis. A P-value ≤0.05 was considered significant for all statistical tests. All calculations were performed with SPSS version 25 (IBM Corp, Armonk, NY).
A total of 167 patients were included in the study. The median age of the cohort was 28 years (range, 16–70), 50.9% (N=85) were male, and AYA represented 67.1% (N=112) of the cases. An ECOG ≤2 was observed in 96.4% (N=161), and 48.5% (N=81) of patients had 1 or more additional comorbidities at diagnosis; of these 21% (N=17) had diabetes, 16.1% (N=13) had hypertension, and 44.4% (N=36) were obese. Regarding ALL diagnosis, immunophenotype was B-cell in 97.6% (N=163) and T-cell in 2.4% (N=4); high-risk cytogenetics were present in 23.3% (N=39). Additional baseline characteristics are presented in Table 1.
Induction therapy with Hyper-CVAD was administered in 65.3% (N=109) of patients, HOP0195/0612 in 28.7% (N=48), and other regimens in 6% (N=10). Flow cytometry demonstrated CD20 positivity in 68.9% (N=115) of cases. A total of 24 patients (20.9%) were receiving rituximab in addition to chemotherapy. The median follow-up was 10 months (range, 10–108). All patients underwent complete remission assessment, and 77.2% (N=129) achieved CR1. Among CD20-positive patients, 79.2% (N=19) of those receiving rituximab reached CR1 as compared to 76.9% (N=70) of those who were not receiving rituximab (P=0.55). There was no difference in CR1 rates when analyzed by age group (P=0.71) or cytogenetic risk stratification (P=0.50).
Induction-related complications were reported in 152 (91%) patients. The most common type of complications were infectious diseases in 87.4% (N=146) of patients, followed by metabolic complications in 46.1% (N=70), and hematologic in 11.1% (N=18). Complication types by subgroup are further described in Table 2. Regarding infectious complications, a bacterial isolate was obtained in 69.9% (N=102) of cases, most of which were gram-negative rods (N=72) followed by gram-positive cocci (N=20). Invasive fungal infections (IFIs) represented 15 cases of Aspergillus sp. and 14 of Candida sp. A total of 46 patients developed shock during the course of induction therapy, and 85.7% (N=39) was related to sepsis.
IRM in our cohort was 12% (N=20). The cause of death was an infectious complication in 14 patients (77.8%), a hemorrhagic complication in 3 patients (16.7%), and 3 patients (16.7%) had other causes of death. On univariate analysis, the factors related to a decreased OS after induction included the following: CNS status at diagnosis (P<0.001), TLS (P=0.005), DIC (P=0.037), shock (P<0.001), bloodstream infection (P=0.020), requirement of renal replacement therapy (P<0.001), and requirement of assisted invasive mechanical ventilation (P<0.001). The presence of comorbidities, including diabetes, hypertension, and obesity, were not associated with an increased IRM (P=0.49) (Table 3). The IRM of AYAs and older adults was 11.6% vs. 12.7% (P=0.83), respectively, with no difference in the causes of death. According to cytogenetics, there were no differences in mortality between the groups of normal karyotype, Ph+, complex karyotype, and other abnormalities (11.1% vs. 4.3% vs. 16.7% vs. 6.7%, respectively; P=0.68). Leukocytosis, considering cutoff values according to leukemia phenotype, did not affect IRM (15.8% vs. 11.0%; P=0.43).
Current reports on survival outcomes for ALL have particularly focused on long-term mortality of patients from developed nations and pediatric cohorts; thus, information on IRM and its risk factors is scarce. In this setting, adults in LMICs are particularly faced with challenges associated with limitations in infrastructure and resources that hinder accessibility to molecular diagnosis, pediatric-inspired regimens, and supportive measures required for appropriate patient management during the post-induction period. To the best of our knowledge, this is the first report evaluating mortality and risk factors for IRM in an adult population living in a LMIC.
Our cohort’s baseline demographics closely resemble those of other Latin American countries in terms of age distribution, CNS status, and PS, where a young median age and a high PS at diagnosis are expected. Patients in the AYA group, which in this study comprised more than 50%, have been related with a worse prognosis. This is mainly attributed to the biology of disease, when compared with other age groups. Particularly in our group, where the prevalence of comorbidities was high, we had a low SES, and 25% of high risk cytogenetics showed no impact on IRM rates [16].
In this cohort, we report an IRM of 12% and a CR1 of 77.2%, which are within the reported rates from other ALL groups [14]. No significant difference for IRM or CR1 was observed when comparing the different induction regimens used, including those in which rituximab was added because of CD20 positivity. These findings are consistent with those of published reports, which shows improved disease-free survival and CR1 duration with rituximab use, but no direct impact on short-term outcomes such as those evaluated in the present study [17].
As expected for an LMIC, we report a high rate of complications during the induction therapy, where infection is the most common. Infections with proven bacterial foci and neutropenic fever were similar to those reported in pediatric and adult cohorts with the use of intensive regimens [5, 18]. Remarkably, the prevalence of IFI in our cohort was significantly higher than expected, closely resembling IFI trends observed in patients with myeloid malignancies [19-21]. The explanation to this was most likely multifactorial and associated with the living situation of patients, limited spectrum of prophylactic antifungals, and renovations on hospital grounds. Cognizance is raised to the fact that IFIs did not significantly increase IRM, yet the long-term impact on clinical outcomes was not evaluated.
The 2 factors associated with an increased IRM on multivariate analysis were dialysis requirements and CNS status. With end-organ failure, dialysis requirement serves as a proxy to multiple systemic processes including, but not limited to TLS, tumor burden, and drug toxicity. Similar to the results observed in acute kidney injury during acute myeloid leukemia induction therapy [22], the survival of patients requiring hemodialysis in our cohort was favorable only for those achieving CR1, contrary to the 5 patients not achieving this response and who died prior to day +60. The influence of CNS status on long-term outcomes and mortality in ALL has been well described in the literature [23, 24]. The role of this factor and its relationship with IRM has been far less studied, but possible explanations include greater leukemic burden and the proliferation of far more aggressive leukemic phenotypes.
We acknowledge that the retrospective nature, sample size, and lack of molecular profile assessment in our study are important limitations; nevertheless, the particularities of ALL in Latin America urges the need to analyze setting-specific data to promote the adaptation of evidence-based medicine in the current clinical practice and resources of hematologists. This and other real-world studies set the base for further research in the field of adult ALL in LMICs.
In conclusion, this study supports previous data showing that in Latin America, adult ALL patients treated with intensive induction regimens have higher IRM and complication rates than those in high-income countries. A higher mortality rate in our population was mainly related to patients presenting with end-stage organ disease and higher tumor burden at diagnosis. In the future, prospective studies that include pediatric-appropriate regimens and molecular diagnoses are warranted to further analyze possible factors that can predict IRM in this particular group of patients.
REFERENCES
1. Terwilliger T, Abdul-Hay M. 2017; Acute lymphoblastic leukemia: a comprehensive review and 2017 update. Blood Cancer J. 7:e577. DOI: 10.1038/bcj.2017.53. PMID: 28665419. PMCID: PMC5520400.

2. Larson RA, Dodge RK, Burns CP, et al. 1995; A five-drug remission induction regimen with intensive consolidation for adults with acute lymphoblastic leukemia: cancer and leukemia group B study 8811. Blood. 85:2025–37. DOI: 10.1182/blood.V85.8.2025.bloodjournal8582025. PMID: 7718875.

3. Wheeler K, Chessells JM, Bailey CC, Richards SM. 1996; Treatment related deaths during induction and in first remission in acute lymphoblastic leukaemia: MRC UKALL X. Arch Dis Child. 74:101–7. DOI: 10.1136/adc.74.2.101. PMID: 8660070. PMCID: PMC1511507.

4. Ramos C, Rozen E, León M, et al. 2011; Results of treatment of acute lymphoblastic leukemia in two cohorts of Mexican patients. Rev Med Chil. 139:1135–42. DOI: 10.4067/S0034-98872011000900004. PMID: 22215391.
5. Fernandes da Silva Junior W, Medina AB, Yamakawa PE, Buccheri V, Velloso EDRP, Rocha V. 2018; Treating adult acute lymphoblastic leukemia in Brazil-increased early mortality using a German multicenter acute lymphoblastic leukemia-based regimen. Clin Lymphoma Myeloma Leuk. 18:e255–9. DOI: 10.1016/j.clml.2018.03.001. PMID: 29605423.
6. Chessells JM, Hall E, Prentice HG, Durrant J, Bailey CC, Richards SM. 1998; The impact of age on outcome in lymphoblastic leukaemia; MRC UKALL X and XA compared: a report from the MRC Paediatric and Adult Working Parties. Leukemia. 12:463–73. DOI: 10.1038/sj.leu.2400959. PMID: 9557602.

7. Roberts KG. 2018; Genetics and prognosis of ALL in children vs adults. Hematology Am Soc Hematol Educ Program. 2018:137–45. DOI: 10.1182/asheducation-2018.1.137. PMID: 30504302. PMCID: PMC6245970.

8. Pichler H, Reismüller B, Steiner M, et al. 2013; The inferior prognosis of adolescents with acute lymphoblastic leukaemia (ALL) is caused by a higher rate of treatment-related mortality and not an increased relapse rate--a population-based analysis of 25 years of the Austrian ALL-BFM (Berlin-Frankfurt-Münster) Study Group. Br J Haematol. 161:556–65. DOI: 10.1111/bjh.12292. PMID: 23480776.

9. Gupta S, Antillon FA, Bonilla M, et al. 2011; Treatment-related mortality in children with acute lymphoblastic leukemia in Central America. Cancer. 117:4788–95. DOI: 10.1002/cncr.26107. PMID: 21446043.

10. Slats AM, Egeler RM, van der Does-van den Berg A, et al. 2005; Causes of death--other than progressive leukemia--in childhood acute lymphoblastic (ALL) and myeloid leukemia (AML): the Dutch Childhood Oncology Group experience. Leukemia. 19:537–44. DOI: 10.1038/sj.leu.2403665. PMID: 15690069.

11. Oken MM, Creech RH, Tormey DC, et al. 1982; Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 5:649–55. DOI: 10.1097/00000421-198212000-00014. PMID: 7165009.

12. Swerdlow SH, Campo E, Pileri SA, et al. 2016; The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 127:2375–90. DOI: 10.1182/blood-2016-01-643569. PMID: 26980727. PMCID: PMC4874220.

13. Brown PA, Wieduwilt M, Logan A, et al. 2019; Guidelines Insights: Acute Lymphoblastic Leukemia, Version 1.2019. J Natl Compr Canc Netw. 17:414–23. DOI: 10.6004/jnccn.2019.0024. PMID: 31085755.
14. Kantarjian HM, O'Brien S, Smith TL, et al. 2000; Results of treatment with hyper-CVAD, a dose-intensive regimen, in adult acute lymphocytic leukemia. J Clin Oncol. 18:547–61. DOI: 10.1200/JCO.2000.18.3.547. PMID: 10653870.

15. Arteaga-Ortiz L, Buitrón-Santiago N, Rosas-López A, et al. 2008; Acute lymphoblastic leukemia: experience in adult patients treated with hyperCVAD and 0195 Protocol, at the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán. Cohort 2003-2007. Rev Invest Clin. 60:459–69. PMID: 19378832.
16. Ribera JM, Oriol A. 2009; Acute lymphoblastic leukemia in adolescents and young adults. Hematol Oncol Clin North Am. 23:1033–42. viDOI: 10.1016/j.hoc.2009.07.002. PMID: 19825451.

17. Thomas DA, O'Brien S, Kantarjian HM. 2009; Monoclonal antibody therapy with rituximab for acute lymphoblastic leukemia. Hematol Oncol Clin North Am. 23:949–71. vDOI: 10.1016/j.hoc.2009.07.005. PMID: 19825447. PMCID: PMC4458386.

18. Jaime-Pérez JC, Jiménez-Castillo RA, Herrera-Garza JL, Gutiérrez-Aguirre H, Marfil-Rivera LJ, Gómez-Almaguer D. 2017; Survival rates of adults with acute lymphoblastic leukemia in a low-income population: a decade of experience at a single institution in Mexico. Clin Lymphoma Myeloma Leuk. 17:60–8. DOI: 10.1016/j.clml.2016.08.013. PMID: 27600987.

19. Lepe-Zuniga JL, Ramirez-Nova V. 2019; Elements associated with early mortality in children with B cell acute lymphoblastic leukemia in Chiapas, Mexico: a case-control study. J Pediatr Hematol Oncol. 41:1–6. DOI: 10.1097/MPH.0000000000001337. PMID: 30339656.

20. Mariette C, Tavernier E, Hocquet D, et al. 2017; Epidemiology of invasive fungal infections during induction therapy in adults with acute lymphoblastic leukemia: a GRAALL-2005 study. Leuk Lymphoma. 58:586–93. DOI: 10.1080/10428194.2016.1204652. PMID: 27397551.

21. Lehrnbecher T. 2012; Infectious complications in acute lymphoblastic leukemia: individually tailored prevention and treatment. Pharm Unserer Zeit. 41:228–33. DOI: 10.1002/pauz.201200472. PMID: 22844670.
22. Lahoti A, Kantarjian H, Salahudeen AK, et al. 2010; Predictors and outcome of acute kidney injury in patients with acute myelogenous leukemia or high-risk myelodysplastic syndrome. Cancer. 116:4063–8. DOI: 10.1002/cncr.25306. PMID: 20564156. PMCID: PMC4181579.

23. Thomas X, Le QH. 2008; Central nervous system involvement in adult acute lymphoblastic leukemia. Hematology. 13:293–302. DOI: 10.1179/102453308X343374. PMID: 18854093.

24. Lazarus HM, Richards SM, Chopra R, et al. 2006; Central nervous system involvement in adult acute lymphoblastic leukemia at diagnosis: results from the international ALL trial MRC UKALL XII/ECOG E2993. Blood. 108:465–72. DOI: 10.1182/blood-2005-11-4666. PMID: 16556888. PMCID: PMC1895498.

Fig. 1
Cumulative mortality risk related to (A) central nervous system status and (B) dialysis requirement.
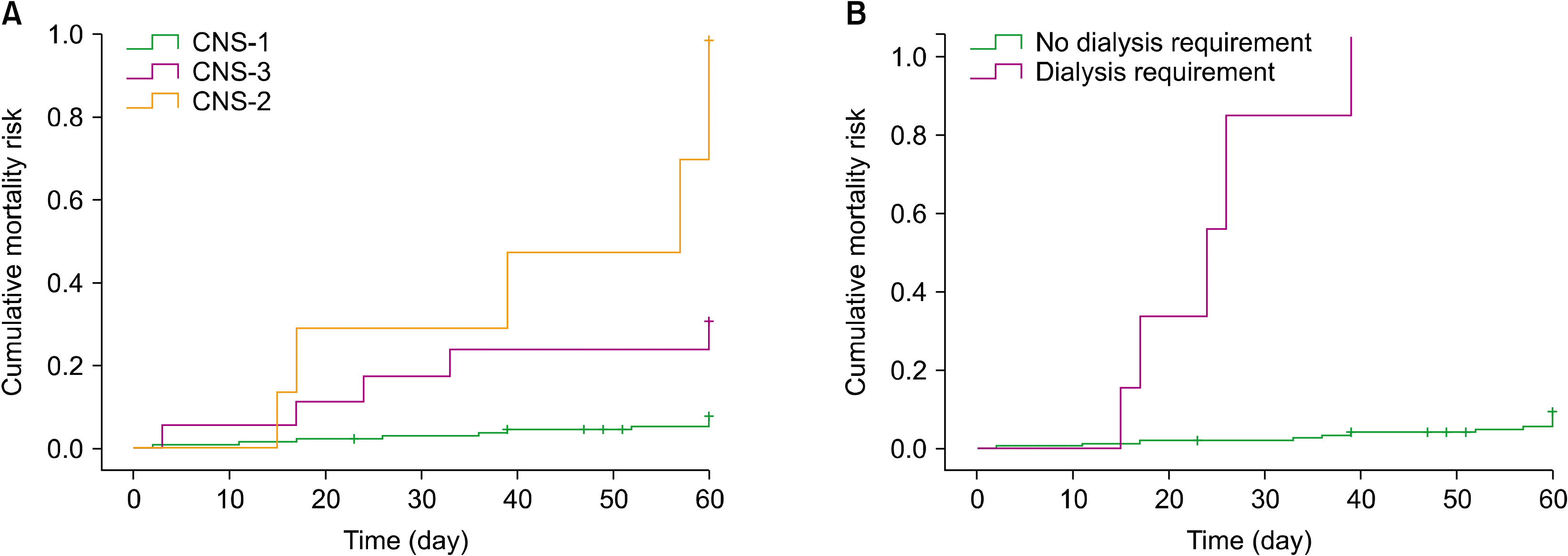
Table 1
Patients baseline characteristics grouped by AYA and non-AYA.
| Characteristics | AYA (N=112) | Non-AYA (N=55) |
|---|---|---|
| Male sex, N (%) | 59 (52.7) | 26 (47.3) |
| Age, median (range) | 22 (16–39) | 51 (40–70) |
| Socioeconomic status, N (%) | ||
| Low-income | 111 (99.1) | 47 (85.5) |
| Middle-income | 0 (0) | 3 (5.4) |
| High-income | 1 (0.9) | 5 (9.1) |
| ECOG ≤2, N (%) | 109 (97.3) | 52 (94.5) |
| Comorbidities, N (%) | 45 (40.2) | 36 (65.5) |
| DM | 2 (1.8) | 15 (27.3) |
| HTN | 4 (3.6) | 9 (16.4) |
| Obesity | 21 (18.8) | 15 (27.3) |
| ALL phenotype, N (%) | ||
| B-cell leukemia | 109 (97.3) | 54 (98.2) |
| Pre-B | 102 (93.6) | 54 (100) |
| Mature B | 6 (5.5) | 0 (0) |
| Pro-B | 1 (0.9) | 0 (0) |
| T-cell leukemia | 3 (2.7) | 1 (1.8) |
| Cytogenetic abnormalitiesa), N (%) | 28 (25.5) | 20 (38.5) |
| Philadelphia chromosome | 17 (15.5) | 12 (23.1) |
| MLL rearrangements | 1 (0.9) | 1 (1.9)c) |
| Hypodiploidy | 0 (0) | 2 (3.9) |
| Complex karyotype | 5 (4.6)b) | 1 (1.9) |
| Others | 8 (7.3) | 5 (9.6) |
| CNS involvement, N (%) | 13 (11.6) | 6 (10.9) |
Table 2
Induction-related complications.
Table 3
Factors related to a decreased overall survival after induction.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download