Abstract
Despite the availability of therapies to treat patients with immune thrombocytopenia (ITP), there is currently little data from randomized trials to assist clinicians in managing patients. The evidence-based guidelines of the Korean Society of Hematology Aplastic Anemia Working Party (KSHAAWP) are intended to support patients and physicians in the management of ITP. Experts from the KSHAAWP discussed and described this guideline according to the current treatment situation for ITP in Korea and finalized the guidelines. The expert panel recommended the management of ITP in adult and pediatric patients with newly diagnosed, persistent, and chronic disease refractory to first-line therapy with minor bleeding. Management approaches include observation and administration of corticosteroids, intravenous immunoglobulin, anti-D immunoglobulin, and thrombopoietin receptor agonists. Currently, evidence supporting strong recommendations for various management approaches is lacking. Therefore, a large focus was placed on shared decision-making, especially regarding second-line treatment.
These guidelines aimed to provide helpful recommendations for managing adult and pediatric patients with immune thrombocytopenic purpura (ITP). In addition, these guidelines aim to provide clinical support for the decision-making process regarding different treatment courses.
ITP is an acquired autoimmune disorder characterized by low platelet count resulting from platelet destruction and impaired production. The incidence of ITP in Western countries is 2–5 per 100,000 person-years [1-5]. In national studies using the Korea Health Insurance Research and Assessment (HIRA) database, the incidence rate of ITP for all ages is 5.3 per 100,000 person-years, while it is 13.39 and 18.1 per 100,000 person-years for children aged <18 years [6-8]. ITP can be an isolated primary event or secondary to other clinical conditions. ITP is a heterogeneous disorder with variable clinical symptoms and signs and remains a diagnosis of exclusion of other causes of thrombocytopenia [9]. The clinical course of ITP may also vary depending on whether it is primary ITP (not associated with other conditions), occurring in the setting of autoimmune cytopenia (Evans syndrome), a manifestation of primary immunodeficiency, or is associated with autoimmune or infectious causes (secondary ITP). In secondary ITP, treatment is often directed towards managing underlying causes.
Bleeding episodes are often unpredictable, and patients with ITP, even in severe thrombocytopenia, may not have bleeding, except bruising and petechiae [10-12]. However, severe bleeding may occur [11-13]. Serious bleeding was reported in 9.5% [95% confidence interval (CI), 4.1–17.1] of adults [11]. Adults with ITP have a 1.3–2.2-fold higher mortality rate than the general population due to cardiovascular events, infectious diseases, and bleeding episodes [14]. In addition, ITP has a significant impact on health-related quality of life (HRQoL) [15, 16].
Whether a patient can be observed without treatment or requires further treatment is complex and varies based on comorbidities, medications, and age, all of which affect the risk of bleeding [17, 18]. In addition, management approaches may vary according to the duration of the disease, accessibility to care, quality of life implications, and preferences of the patient and clinicians. Considering the inter-patient variability in the pathophysiology of immune dysregulation and the lack of effective predictors of treatment response, the choice of appropriate therapy may vary significantly among physicians when the treatment has been decided [19].
For the 2022 update, an expert panel reviewed the evidence published since the 2017 Korean recommendation [20]. In these guidelines, the expert panel recommended valuable principles for managing adult and pediatric patients with ITP based on evidence and expert opinions.
In adult patients with newly diagnosed ITP and a platelet count <20×109/L without symptoms or with minor mucocutaneous bleeding, we recommend corticosteroids rather than observation.
To choose corticosteroid versus observation, physicians should consider the level of platelet count, additional comorbidities, use of anticoagulant or antiplatelet agents, need for subsequent procedures, and patient age.
The benefits cannot be estimated from the data because of the lack of direct comparison results [21-27]. The response rate of the platelet count at 7 days was 55.8% with corticosteroids; however, the overall remission rate was relatively low (30.2%) [21]. The harms and burdens could not be precisely estimated from the data because of the lack of direct comparison results. Undesirable adverse effects of observation exist in this setting, considering that thrombocytopenia is a surrogate for future bleeding events and treatment failure in adult patients. Bleeding episodes (3.3%) and mortality (5.7%) were only reported in the corticosteroid-treated group [26].
In adult patients with newly diagnosed ITP and a platelet count ≥20×109/L without symptoms or minor mucocutaneous bleeding, we recommend observation rather than corticosteroids. For patients with a platelet count at the lower end of this threshold, those with additional comorbidities, anticoagulant or antiplatelet agents, or need to follow the procedures. Corticosteroid treatment may be appropriate for elderly patients (aged >60 yr).
The benefit cannot be precisely estimated from the data because of the lack of direct comparison results. However, major bleeding episodes were not different and low in both arms (corticosteroids vs. observation: 0.9% vs. 0%) [28-32]. Based on indirect evidence, the side effects of corticosteroids are not trivial; therefore, the undesirable adverse effects of corticosteroids are moderate.
In adult patients with newly diagnosed ITP, we recommend a short course (≤6 wk) of prednisone rather than a prolonged course (>6 wk, including treatment and tapering).
No studies supporting short courses of prednisone are currently available, and this recommendation is based on expert experience [33-35]. It is presumed that a trivial benefit exists in continuing corticosteroids for more than 6 weeks, and many patients require additional treatment. For patients requiring further treatment, an alternative therapy is preferable to continued corticosteroid exposure. The likelihood of harm and risk of adverse events was enormous with the continuation of corticosteroids for more than 6 weeks. Adverse events included hypertension, hyperglycemia, sleep and mood disturbances, epigastric soreness, ulcer formation, glaucoma, myopathy, and osteoporosis.
In adult patients with newly diagnosed ITP, we recommend either prednisone (0.5–2.0 mg/kg/day) or dexamethasone (40 mg/day for 4 days) as the type of initial corticosteroid treatment. If we stress the rapidity of the platelet count response, dexamethasone may be preferable to prednisone, considering that the response at 7 days was more desirable with dexamethasone.
Randomized study data showed an increased platelet count response at 7 days to dexamethasone [relative risk (RR), 1.31; 95% CI, 1.11–1.54] [21-23]. The remission rate was higher among the dexamethasone-treated patients than with prednisone (RR, 2.96; 95% CI, 1.03–8.45); however, the confidence level was low because the definition of remission applied by the trials was indirect, and the dose of corticosteroid was heterogeneous [21-23, 25, 28, 36, 37]. No clear benefit was found regarding the 1 month response rate, durable response rate, or incidence of major bleeding episodes. The duration of the initial response following a cycle of dexamethasone varied. We also recommend that the platelet count should be monitored frequently.
Despite the lack of direct evidence, the risk of adverse events in clinical practice varies according to the dose and duration of corticosteroid treatment, comorbidities, and patient age. Concerns regarding dexamethasone in patients with underlying diabetes and the elderly (aged >60 yr) also exist.
In adult patients with newly diagnosed ITP, we recommend corticosteroids alone rather than corticosteroids combined with rituximab as the initial treatment. An initial course of corticosteroids combined with rituximab may be preferable when the possibility of remission is higher than the concerns regarding the potential side effects of rituximab.
Moderate effects were observed with concomitant corticosteroids and rituximab, particularly a higher durable response rate (RR, 1.70; 95% CI, 1.34–2.16) and remission rate (RR, 1.58; 95% CI, 1.00–2.52) [38-40]. No difference was observed regarding the impact on the response rate at 1 month, prevention of major bleeding episodes or mortality, and no data on HRQoL. The certainty level in the evidence for benefits was extremely low due to the absence of HRQoL data, unknown and non-standardized corticosteroid doses for comparison, and the absence of long-term follow-up results. The prioritized outcome of infection was not different between the two treatments, even though the CI was significant (RR, 3.18; 95% CI, 0.13–76.25).
In adult patients with corticosteroid-dependent or refractory ITP for more than 3 months, we recommend a thrombopoietin receptor agonist (TPO-RA), either eltrombopag or romiplostim. Physicians should consider the preferences of individual patients when choosing daily oral medications or weekly subcutaneous injections.
A comparison was made between the durable response rates of eltrombopag and romiplostim (odds ratio=0.20; 95% CI, 0.01–2.13) [41-43]. The major bleeding rates and discontinuation or reduction rates of corticosteroids could not be estimated from the data because of a lack of comparisons. However, the desirable effects were minimally different. There were no significant differences in the outcomes, including durable response, bleeding, and corticosteroid discontinuation or reduction rates. The undesirable effects are trivial. Elevation of alanine/aspartate transaminase related to eltrombopag was mild and reversible in most participants; therefore, it did not affect the balance of undesirable events. No net health benefits or harmful differences related to eltrombopag or romiplostim were observed. Based on the available evidence, it is presumed that there is no difference between the two treatments. Preference of the patients for the route of administration – oral daily medication compared with weekly subcutaneous injection– likely affects treatment decision-making.
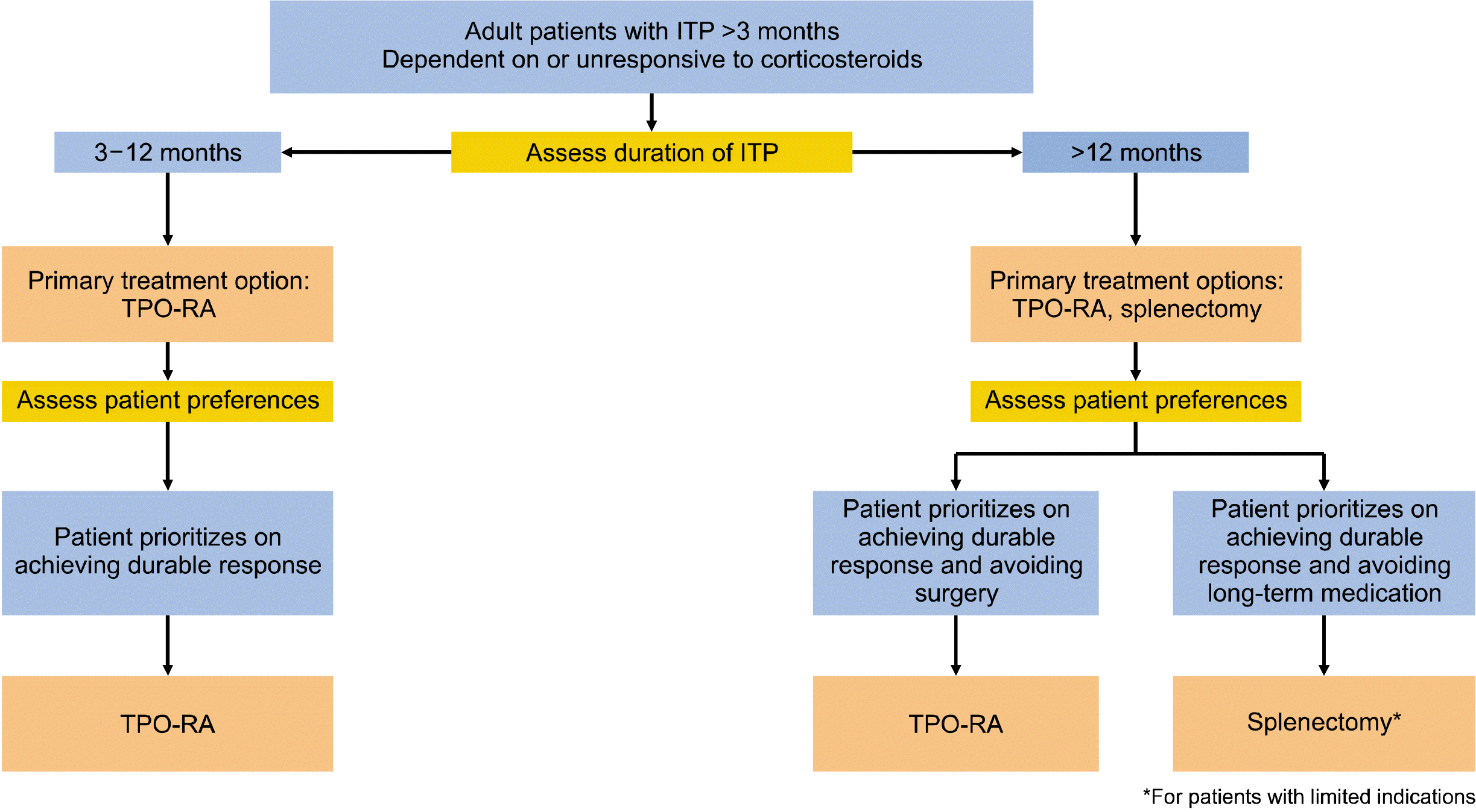
In adult patients with ITP lasting ≥3 months who are corticosteroid-dependent or unresponsive to corticosteroids, we recommend TPO-RA rather than splenectomy. Splenectomy should be postponed for at least 12 months after diagnosis because of the possibility of spontaneous remission in the first year. For patients with ITP lasting >12 months, a splenectomy can only be performed in those with limited indications.
The American Society of Hematology (ASH) guidelines suggest either splenectomy or TPO-RA for corticosteroid-dependent patients or do not respond to corticosteroids [44]. Despite the lack of direct evidence, both treatment options are associated with a more durable response. No difference in major bleeding was observed between the patients treated with splenectomy and those treated with TPO-RAs (4.6% and 3.5%, respectively). As there is no single treatment optimal for all patients with ITP, treatment should be individualized based on the duration of ITP, age of the patient, medical condition, and patient preferences [44].
For patients with ITP of 3–12 months, because of the possibility of spontaneous remission in the first year, it is recommended to postpone splenectomy to 12 months from diagnosis [45-50]. For such patients, TPO-RA is the primary treatment option because of its greater response durability. Splenectomy and TPO-RA can be viable options for patients with ITP lasting >12 months [51-66]. A splenectomy is an option for patients who prioritize durable responses and avoid long-term treatment. Currently, the use of splenectomy as a second-line treatment for ITP is gradually decreasing. In a Korean ITP study, only 3% of treated patients received splenectomy as a second-line treatment [6]. This low incidence of splenectomy for ITP results from high post-splenectomy morbidity and mortality related to the operation and long-term complications, such as infection, cardiovascular events, and venous thromboembolism, especially in older patients [6]. Alternative effective treatment options, such as TPO-RA, are also available for adult nonsplenectomized patients who have a medically unfit condition to splenectomy, as suggested by the Korean ITP guidelines in 2017. Unlike the ASH guidelines, the International Consensus Report (ICR) guidelines recommend splenectomy only after medical treatment failure [67]. Based on the current data, splenectomy should be performed under limited indications considering the age, comorbidities that can worsen the risk of surgery, and patient preference [activity levels, occupation, need for procedures, acceptance of minor bleeding, the persistence of treatment duration (chronic therapy vs. limited therapy), preference for daily tablets or weekly injections, and financial ability]. Young patients with an active lifestyle, including those who participate in high-risk activities, may prefer splenectomies. In addition, other patients who do not comply with the medication (dietary restriction for eltrombopag and weekly injection of romiplostim) may consider splenectomy. For patients who prefer medical treatment and prioritize avoiding surgery, a suitable option is the TPO-RA. The common adverse effects of TPO-RA are gastrointestinal symptoms, mild transaminase elevation, and headache, most of which are mild. Bone marrow fibrosis is a potential side effect of concern with the use of TPO-RAs; however, the risk of clinically meaningful fibrosis seems to be low. Thrombotic events of TPO-RAs should be considered in patients with ITP with significant risk factors for venous and arterial thrombosis. In addition, the disadvantage of a relatively high cost due to the long-term use of a TPO-RA should be considered when discussing treatment options. Most importantly, for patients who prioritize achieving a durable response, the best option is TPO-RA. An individualized approach for selecting second-line treatment based on ITP duration and patient preference is shown in Fig. 1.
In pediatric patients with newly diagnosed ITP without bleeding or with minor bleeding, observation rather than corticosteroids is recommended.
There was no perceived benefit of corticosteroids in terms of durable platelet response (78.5% with corticosteroids and 87.3% with observation), remission (76.6% with corticosteroids and 63.6% with observation), or reduction in major bleeding (0% for both treatments) in this setting [68-69]. In addition, the undesirable effects of corticosteroids increase in proportion to the treatment duration.
In pediatric patients with newly diagnosed ITP without bleeding or with minor bleeding, we recommend observation rather than intravenous immunoglobulin (IVIG) or anti-D immunoglobulin.
A randomized trial of IVIG over observation showed no differences in outcomes at 12 months and durable response. The incidence of bleeding and mortality was similar between the two groups (0.6% and 1.8% with IVIG and 0% and 0% with observation for bleeding and mortality, respectively) [69-73]. Although there was a lack of direct comparisons, there was only a small benefit from anti-D immunoglobulin. No data are available on major bleeding and mortality associated with anti-D immunoglobulins. IVIG has side effects, such as infusion-related symptoms, thrombosis, and acute renal failure, and anti-D immunoglobulins such as intravascular hemolysis [69-73].
We recommend 7 days or shorter courses of corticosteroids rather than longer than 7 days in pediatric patients with newly diagnosed ITP with non-life-threatening mucosal bleeding and diminished HRQoL. In addition, we recommend 2–4 mg/kg/day of prednisolone (maximum 120 mg/day) for 5–7 days, rather than 0.6 mg/kg/day of dexamethasone (maximum 40 mg/day) for 4 days.
Given the low rate of bleeding, high rate of spontaneous remission, overall low morbidity in the pediatric population, and lack of evidence for the benefit of long-term corticosteroids, there was likely a small benefit in continuing corticosteroids for longer than 7 days [44]. A longer course of corticosteroids (>7 days) is likely to increase the risk of adverse events, resulting in poor treatment adherence in this population. In the absence of increased benefits with a longer course of corticosteroids and the side effects associated with prolonged corticosteroid exposure, we recommend that the balance of effects favored 7 days or shorter course of corticosteroids over longer periods [44]. There are limited data on treatment with dexamethasone compared with prednisolone in the pediatric population. Higher corticosteroid doses of dexamethasone used in adult trials are deemed potentially intolerable by some pediatric patients with regard to short-term side effects. In the absence of data, there is no strong evidence suggesting that dexamethasone is superior to prednisolone.
In pediatric patients with newly diagnosed ITP with non-life-threatening bleeding and diminished HRQoL, we recommend corticosteroids rather than anti-D immunoglobulins or IVIG.
Based on randomized trial data, only trivial benefits were observed with IVIG compared with corticosteroids regarding durable response, remission rate, prevention of bleeding events, and mortality [44, 70-73]. In addition, a short course of corticosteroids is usually associated with mild side effects in most pediatric patients. However, some concerns related to anti-D immunoglobulins and IVIG, leading to the need for additional medical interventions.
In pediatric patients with ITP with non-life-threatening mucosal bleeding and diminished HRQoL who do not respond to first-line treatment, we recommend using TPO-RAs rather than rituximab or splenectomy.
Although there are no data available for direct comparison of TPO-RAs with either rituximab or splenectomy, TPO-RAs show a moderate benefit over rituximab and splenectomy in various studies of pediatric patients with ITP who are unresponsive to first-line treatment [44, 74-76]. Compared with rituximab and splenectomy, TPO-RAs provide a stable long-term platelet response and reduce bleeding events [74-76]. However, there is concern about developing persistent hypogammaglobulinemia after rituximab treatment in the pediatric population [44, 74-76]. In addition, operative complications associated with splenectomy were identified in 5.9% of children [44]. However, thrombosis was not observed in any children [44].
In adult patients with ITP who respond to TPO-RAs, we recommend using the lowest dose of TPO-RAs, sufficient to maintain a platelet count ≥50×109/L.
TPO-RAs are generally used as a maintenance treatment for ITP. However, the optimal dose of TPO-RAs and the platelet count target necessary to maintain response and reduce bleeding in responders to TPO-RAs differ slightly depending on the study [77, 78]. In published studies on romiplostim, most adult patients who responded to romiplostim achieved and maintained a platelet count ≥50×109/L with a median dose of 2 mcg/kg (up to 10 mcg/kg) [78]. In prescribed information of two TPO-RAs, the lowest dose of TPO-RAs was recommended to achieve and maintain a platelet count ≥50×109/L as necessary to reduce the risk for bleeding. In a single-arm phase II study of romiplostim in adults with primary ITP who had received first-line therapy (remission study), 75 patients who achieved a response started tapering and discontinuation of romiplostim [79]. Patients with a platelet count ≥50×109/L at 12 months started to receive a dose taper, in which the romiplostim dose was decreased, and the platelet count was maintained. In this study, 32% (24/75) of patients who discontinued romiplostim maintained platelet count ≥50×109/L without any additional treatment for 24 consecutive weeks.
Rituximab is a monoclonal antibody against the CD20 antigen that targets the B cell-producing antibodies for platelets. Rituximab is usually administered at 375 mg/m2 intravenously every 4 weeks. In adults with ITP who fail to respond to TPO-RA or experience relapse after discontinuing TPO-RA, rituximab can be administered as a third-line therapy [80].
Azathioprine is administered at an oral dose of 50–200 mg/day in adult patients and is sometimes administered with danazol; however, there is little data to support an improved response to the combination. It takes several months to have a full effect on ITP. Azathioprine is one of the drugs deemed “safe” for patients with ITP in pregnancy, without increased risk of fetal malformation, and safe during lactation. Major adverse events included nausea, infection, liver function abnormalities, neutropenia, and anemia [81].
Cyclophosphamide is a chemotherapeutic agent that has been used since 1959 to treat malignant disease at high doses and as an immunosuppressive agent to treat autoimmune disorders at low doses. Cyclophosphamide is usually delivered as an oral dose of 50–200 mg/day for adult patients. Major adverse events include bone marrow suppression, infection, infertility, secondary malignancies, and hemorrhagic cystitis. However, its use is contraindicated during pregnancy and lactation [82].
Cyclosporine A levels were adjusted by monitoring drug levels. However, the usual starting dose is 3–6 mg/kg/day, with a maximum dose of 200 mg for adult patients. Major adverse events were gingival hyperplasia, hypertension, renal toxicity, and emesis. Therefore, its use is contraindicated during pregnancy and lactation [83].
Danazol is usually administered at an oral dose of 200–800 mg/day in adults. Its androgenic effects are related to major adverse events (especially in women), transaminitis, weight gain, acne, rash, mood changes, amenorrhea, and virilization. Therefore, clinicians should perform liver function tests at least once a month. However, they are contraindicated during pregnancy and lactation. It has sometimes been used in combination with azathioprine, but there is little evidence to support the added benefits of this combination [84].
Dapsone is administered orally at 50–100 mg/day to both adult and pediatric patients. The treatment was generally well-tolerated. However, mild hemolysis occurs in most patients, whereas significant hemolysis is less common. Therefore, clinicians should monitor for the potential development of methemoglobin [85-89].
Mycophenolate mofetil is administered orally at 500–2,000 mg/day to adult patients. Serious adverse events include diarrhea, neutropenia, anemia, and viral infections. Prolonged drug use increases the risk for malignancy and progressive multifocal leukoencephalopathy. It has also been associated with pure red aplasia. It is a teratogen that should not be prescribed during pregnancy or lactation [90-92].
Vinca alkaloids can be used as treatment options for ITP. Patients can achieve a rapid response at 7 days with vincristine (1–2 mg per dose once weekly for 2–4 wk in adult patients) or vinblastine (10 mg per dose once weekly for 1–3 wk in adult patients). Almost all patients experience adverse events, such as vincristine neuropathy, vinblastine-associated bone marrow suppression, constipation, hyponatremia, and infusion site vesication. Vinca alkaloids are contraindicated in pregnancy and lactation [93-95].
REFERENCES
1. Abrahamson PE, Hall SA, Feudjo-Tepie M, Mitrani-Gold FS, Logie J. 2009; The incidence of idiopathic thrombocytopenic purpura among adults: a population-based study and literature review. Eur J Haematol. 83:83–9. DOI: 10.1111/j.1600-0609.2009.01247.x. PMID: 19245532.

2. Schoonen WM, Kucera G, Coalson J, et al. 2009; Epidemiology of immune thrombocytopenic purpura in the General Practice Research Database. Br J Haematol. 145:235–44. DOI: 10.1111/j.1365-2141.2009.07615.x. PMID: 19245432.

3. Segal JB, Powe NR. 2006; Prevalence of immune thrombocytopenia: analyses of administrative data. J Thromb Haemost. 4:2377–83. DOI: 10.1111/j.1538-7836.2006.02147.x. PMID: 16869934.

4. Terrell DR, Beebe LA, Vesely SK, Neas BR, Segal JB, George JN. 2010; The incidence of immune thrombocytopenic purpura in children and adults: a critical review of published reports. Am J Hematol. 85:174–80. DOI: 10.1002/ajh.21616. PMID: 20131303.

5. Yong M, Schoonen WM, Li L, et al. 2010; Epidemiology of paediatric immune thrombocytopenia in the General Practice Research Database. Br J Haematol. 149:855–64. DOI: 10.1111/j.1365-2141.2010.08176.x. PMID: 20377590.

6. Lee JY, Lee JH, Lee H, et al. 2017; Epidemiology and management of primary immune thrombocytopenia: a nationwide population-based study in Korea. Thromb Res. 155:86–91. DOI: 10.1016/j.thromres.2017.05.010. PMID: 28525829.

7. Lim JH, Kim YK, Min SH, Kim SW, Lee YH, Lee JM. 2021; Epidemiology and viral etiology of pediatric immune thrombocytopenia through Korean public health data analysis. J Clin Med. 10:1356. DOI: 10.3390/jcm10071356. PMID: 33806145. PMCID: PMC8037772. PMID: 2161b583ca1c42dcb342194d51cfa654.

8. Park SH, Kwak SG, Kim JY. 2021; Incidence and prevalence of immune thrombocytopenia under the copayment waiver policy for pediatric patients in Korea: data from the National Health Claims Database. Lupus. 30:655–60. DOI: 10.1177/0961203321995247. PMID: 33593162.

9. Cines DB, Bussel JB, Liebman HA, Luning Prak ET. 2009; The ITP syndrome: pathogenic and clinical diversity. Blood. 113:6511–21. DOI: 10.1182/blood-2009-01-129155. PMID: 19395674. PMCID: PMC2710913.

10. Neunert CE, Buchanan GR, Blanchette V, et al. 2009; Relationships among bleeding severity, health-related quality of life, and platelet count in children with immune thrombocytopenic purpura. Pediatr Blood Cancer. 53:652–4. DOI: 10.1002/pbc.21978. PMID: 19492316.

11. Neunert C, Noroozi N, Norman G, et al. 2015; Severe bleeding events in adults and children with primary immune thrombocytopenia: a systematic review. J Thromb Haemost. 13:457–64. DOI: 10.1111/jth.12813. PMID: 25495497. PMCID: PMC4991942.

12. Neunert CE, Buchanan GR, Imbach P, et al. 2008; Severe hemorrhage in children with newly diagnosed immune thrombocytopenic purpura. Blood. 112:4003–8. DOI: 10.1182/blood-2008-03-138487. PMID: 18698007. PMCID: PMC2581983.

13. Psaila B, Petrovic A, Page LK, Menell J, Schonholz M, Bussel JB. 2009; Intracranial hemorrhage (ICH) in children with immune thrombocytopenia (ITP): study of 40 cases. Blood. 114:4777–83. DOI: 10.1182/blood-2009-04-215525. PMID: 19767509. PMCID: PMC2786288.

14. Frederiksen H, Maegbaek ML, Nørgaard M. 2014; Twenty-year mortality of adult patients with primary immune thrombo-cytopenia: a Danish population-based cohort study. Br J Haematol. 166:260–7. DOI: 10.1111/bjh.12869. PMID: 24690142.

15. Kuter DJ, Mathias SD, Rummel M, et al. 2012; Health-related quality of life in nonsplenectomized immune thrombocytopenia patients receiving romiplostim or medical standard of care. Am J Hematol. 87:558–61. DOI: 10.1002/ajh.23163. PMID: 22460421.

16. Snyder CF, Mathias SD, Cella D, Isitt JJ, Wu AW, Young J. 2008; Health-related quality of life of immune thrombocytopenic purpura patients: results from a web-based survey. Curr Med Res Opin. 24:2767–76. DOI: 10.1185/03007990802377461. PMID: 18715526.

17. Kühne T, Buchanan GR, Zimmerman S, et al. 2003; A prospective comparative study of 2540 infants and children with newly diagnosed idiopathic thrombocytopenic purpura (ITP) from the Intercontinental Childhood ITP Study Group. J Pediatr. 143:605–8. DOI: 10.1067/S0022-3476(03)00535-3. PMID: 14615730.

18. Li S, Molony JT, Cetin K, Wasser JS, Altomare I. 2018; Rate of bleeding-related episodes in elderly patients with primary immune thrombocytopenia: a retrospective cohort study. Curr Med Res Opin. 34:209–16. DOI: 10.1080/03007995.2017.1360852. PMID: 28748715.

19. Audia S, Mahévas M, Samson M, Godeau B, Bonnotte B. 2017; Pathogenesis of immune thrombocytopenia. Autoimmun Rev. 16:620–32. DOI: 10.1016/j.autrev.2017.04.012. PMID: 28428120.

20. Jang JH, Kim JY, Mun YC, et al. 2017; Management of immune thrombocytopenia: Korean experts recommendation in 2017. Blood Res. 52:254–63. DOI: 10.5045/br.2017.52.4.254. PMID: 29333401. PMCID: PMC5762735.

21. Din B, Wang X, Shi Y, Li Y. 2015; Long-term effect of high-dose dexamethasone with or without low-dose dexamethasone maintenance in untreated immune thrombocytopenia. Acta Haematol. 133:124–8. DOI: 10.1159/000362529. PMID: 25247485.

22. Praituan W, Rojnuckarin P. 2009; Faster platelet recovery by high-dose dexamethasone compared with standard-dose prednisolone in adult immune thrombocytopenia: a prospective randomized trial. J Thromb Haemost. 7:1036–8. DOI: 10.1111/j.1538-7836.2009.03359.x. PMID: 19548911.

23. Mashhadi MA, Kaykhaei MA, Sepehri Z, Miri-Moghaddam E. 2012; Single course of high dose dexamethasone is more effective than conventional prednisolone therapy in the treatment of primary newly diagnosed immune thrombocytopenia. Daru. 20:7. DOI: 10.1186/2008-2231-20-7. PMID: 23351609. PMCID: PMC3557140.

24. Jacobs P, Wood L, Novitzky N. 1994; Intravenous gammaglobulin has no advantages over oral corticosteroids as primary therapy for adults with immune thrombocytopenia: a prospective randomized clinical trial. Am J Med. 97:55–9. DOI: 10.1016/0002-9343(94)90048-5. PMID: 8030657.

25. Wei Y, Ji XB, Wang YW, et al. 2016; High-dose dexamethasone vs prednisone for treatment of adult immune thrombocytopenia: a prospective multicenter randomized trial. Blood. 127:296–302. quiz 370DOI: 10.1182/blood-2015-07-659656. PMID: 26480931.

26. DiFino SM, Lachant NA, Kirshner JJ, Gottlieb AJ. 1980; Adult idiopathic thrombocytopenic purpura. Clinical findings and response to therapy. Am J Med. 69:430–42. DOI: 10.1016/0002-9343(80)90016-9. PMID: 6998293.
27. Houwerzijl EJ, Louwes H, Sluiter WJ, Smit JW, Vellenga E, de Wolf JT. 2008; Platelet production rate predicts the response to prednisone therapy in patients with idiopathic thrombocytopenic purpura. Ann Hematol. 87:975–83. DOI: 10.1007/s00277-008-0537-1. PMID: 18690441.

28. Matschke J, Müller-Beissenhirtz H, Novotny J, et al. 2016; A randomized trial of daily prednisone versus pulsed dexamethasone in treatment-naïve adult patients with immune thrombocytopenia: EIS 2002 study. Acta Haematol. 136:101–7. DOI: 10.1159/000445420. PMID: 27189086.

29. Mazzucconi MG, Francesconi M, Fidani P, et al. 1985; Treatment of idiopathic thrombocytopenic purpura (ITP): results of a multicentric protocol. Haematologica. 70:329–36. PMID: 3935532.
30. Centurioni R, Braianzoni F, Olivieri A, et al. 1990; Treatment of autoimmune thrombocytopenic purpura. Acta Haematol Pol. 21:139–43. PMID: 2131713.
31. Zimmer J, Andrès E, Noel E, Koumarianou A, Blicklé JF, Maloisel F. 2004; Current management of adult idiopathic thrombocytopenic purpura in practice: a cohort study of 201 patients from a single center. Clin Lab Haematol. 26:137–42. DOI: 10.1111/j.1365-2257.2004.00591.x. PMID: 15053808.

32. Bizzoni L, Mazzucconi MG, Gentile M, et al. 2006; Idiopathic thrombocytopenic purpura (ITP) in the elderly: clinical course in 178 patients. Eur J Haematol. 76:210–6. DOI: 10.1111/j.1600-0609.2005.00602.x. PMID: 16412138.

33. Oray M, Abu Samra K, Ebrahimiadib N, Meese H, Foster CS. 2016; Long-term side effects of glucocorticoids. Expert Opin Drug Saf. 15:457–65. DOI: 10.1517/14740338.2016.1140743. PMID: 26789102.

34. Rhen T, Cidlowski JA. 2005; Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. N Engl J Med. 353:1711–23. DOI: 10.1056/NEJMra050541. PMID: 16236742.

35. Schäcke H, Döcke WD, Asadullah K. 2002; Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 96:23–43. DOI: 10.1016/S0163-7258(02)00297-8. PMID: 12441176.

36. Mithoowani S, Gregory-Miller K, Goy J, et al. 2016; High-dose dexamethasone compared with prednisone for previously untreated primary immune thrombocytopenia: a systematic review and meta-analysis. Lancet Haematol. 3:e489–96. DOI: 10.1016/S2352-3026(16)30109-0. PMID: 27658982.

37. Bae SH, Ryoo HM, Lee WS, et al. 2010; High dose dexamethasone vs. conventional dose prednisolone for adults with immune thrombocytopenia: a prospective multicenter phase III trial. Blood (ASH Annual Meeting Abstracts). 116(Suppl):3687. DOI: 10.1182/blood.V116.21.3687.3687.

38. Li Z, Mou W, Lu G, et al. 2011; Low-dose rituximab combined with short-term glucocorticoids up-regulates Treg cell levels in patients with immune thrombocytopenia. Int J Hematol. 93:91–8. DOI: 10.1007/s12185-010-0753-z. PMID: 21188563.

39. Zaja F, Baccarani M, Mazza P, et al. 2010; Dexamethasone plus rituximab yields higher sustained response rates than dexamethasone monotherapy in adults with primary immune thrombocytopenia. Blood. 115:2755–62. DOI: 10.1182/blood-2009-07-229815. PMID: 20130241.
40. Gudbrandsdottir S, Birgens HS, Frederiksen H, et al. 2013; Rituximab and dexamethasone vs dexamethasone monotherapy in newly diagnosed patients with primary immune thrombocytopenia. Blood. 121:1976–81. DOI: 10.1182/blood-2012-09-455691. PMID: 23293082.

41. Cooper K, Matcham J, Helme K, Akehurst R. 2014; Update on romiplostim and eltrombopag indirect comparison. Int J Technol Assess Health Care. 30:129–30. DOI: 10.1017/S0266462313000767. PMID: 24485057.

42. Cooper KL, Fitzgerald P, Dillingham K, Helme K, Akehurst R. 2012; Romiplostim and eltrombopag for immune thrombocytopenia: methods for indirect comparison. Int J Technol Assess Health Care. 28:249–58. DOI: 10.1017/S0266462312000414. PMID: 22980701.

43. Wang L, Gao Z, Chen XP, et al. 2016; Efficacy and safety of thrombopoietin receptor agonists in patients with primary immune thrombocytopenia: a systematic review and meta-analysis. Sci Rep. 6:39003. DOI: 10.1038/srep39003. PMID: 27991534. PMCID: PMC5171907.

44. Neunert C, Terrell DR, Arnold DM, et al. 2019; American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 3:3829–66. DOI: 10.1182/bloodadvances.2019000966. PMID: 31794604. PMCID: PMC6963252.

45. Chater C, Terriou L, Duhamel A, et al. 2016; Reemergence of splenectomy for ITP second-line treatment? Ann Surg. 264:772–7. DOI: 10.1097/SLA.0000000000001912. PMID: 27741009.

46. Kojouri K, Vesely SK, Terrell DR, George JN. 2004; Splenectomy for adult patients with idiopathic thrombocytopenic purpura: a systematic review to assess long-term platelet count responses, prediction of response, and surgical complications. Blood. 104:2623–34. DOI: 10.1182/blood-2004-03-1168. PMID: 15217831.

47. Wang T, Xu M, Ji L, Han ZC, Yang R. 2005; Splenectomy for adult chronic idiopathic thrombocytopenic purpura: experience from a single center in China. Eur J Haematol. 75:424–9. DOI: 10.1111/j.1600-0609.2005.00517.x. PMID: 16191093.

48. Vianelli N, Galli M, de Vivo A, et al. 2005; Efficacy and safety of splenectomy in immune thrombocytopenic purpura: long-term results of 402 cases. Haematologica. 90:72–7. PMID: 15642672.
49. Sampath S, Meneghetti AT, MacFarlane JK, Nguyen NH, Benny WB, Panton ON. 2007; An 18-year review of open and laparoscopic splenectomy for idiopathic thrombocytopenic purpura. Am J Surg. 193:580–3. discussion 583–4. DOI: 10.1016/j.amjsurg.2007.02.002. PMID: 17434359.

50. Gonzalez-Porras JR, Escalante F, Pardal E, et al. 2013; Safety and efficacy of splenectomy in over 65-yrs-old patients with immune thrombocytopenia. Eur J Haematol. 91:236–41. DOI: 10.1111/ejh.12146. PMID: 23679653.

51. Ahmed R, Devasia AJ, Viswabandya A, et al. 2016; Long-term outcome following splenectomy for chronic and persistent immune thrombocytopenia (ITP) in adults and children: splenectomy in ITP. Ann Hematol. 95:1429–34. DOI: 10.1007/s00277-016-2738-3. PMID: 27370992.

52. Zheng CX, Zheng D, Chen LH, Yu JF, Wu ZM. 2011; Laparoscopic splenectomy for immune thrombocytopenic purpura at a teaching institution. Chin Med J (Engl). 124:1175–80. PMID: 21542991.
53. Guan Y, Wang S, Xue F, et al. 2017; Long-term results of splenectomy in adult chronic immune thrombocytopenia. Eur J Haematol. 98:235–41. DOI: 10.1111/ejh.12821. PMID: 27753191.

54. Park YH, Yi HG, Kim CS, et al. 2016; Clinical outcome and predictive factors in the response to splenectomy in elderly patients with primary immune thrombocytopenia: a multicenter retrospective study. Acta Haematol. 135:162–71. DOI: 10.1159/000442703. PMID: 26771656.

55. Li HQ, Zhang L, Zhao H, Ji LX, Yang RC. 2005; Chronic idiopathic thrombocytopenic purpura in adult Chinese patients: a retrospective single-centered analysis of 1791 cases. Chin Med J (Engl). 118:34–7. PMID: 15642223.
56. Montalvo J, Velazquez D, Pantoja JP, Sierra M, López-Karpovitch X, Herrera MF. 2014; Laparoscopic splenectomy for primary immune thrombocytopenia: clinical outcome and prognostic factors. J Laparoendosc Adv Surg Tech A. 24:466–70. DOI: 10.1089/lap.2013.0267. PMID: 24905792.

57. Balagué C, Vela S, Targarona EM, et al. 2006; Predictive factors for successful laparoscopic splenectomy in immune thrombocytopenic purpura: study of clinical and laboratory data. Surg Endosc. 20:1208–13. DOI: 10.1007/s00464-005-0445-6. PMID: 16865623.

58. Wang T, Zhao H, Ren H, et al. 2005; Type 1 and type 2 T-cell profiles in idiopathic thrombocytopenic purpura. Haematologica. 90:914–23. PMID: 15996929.
59. Zheng D, Huang CS, Huang SB, Zheng CX. 2016; Laparoscopic splenectomy for primary immune thrombocytopenia: current status and challenges. World J Gastrointest Endosc. 8:610–5. DOI: 10.4253/wjge.v8.i17.610. PMID: 27668071. PMCID: PMC5027031.

60. Bussel JB, Kuter DJ, George JN, et al. 2006; AMG 531, a thrombopoiesis-stimulating protein, for chronic ITP. N Engl J Med. 355:1672–81. DOI: 10.1056/NEJMoa054626. PMID: 17050891.

61. Bussel JB, Cheng G, Saleh MN, et al. 2007; Eltrombopag for the treatment of chronic idiopathic thrombocytopenic purpura. N Engl J Med. 357:2237–47. DOI: 10.1056/NEJMoa073275. PMID: 18046028.

62. Bussel JB, Provan D, Shamsi T, et al. 2009; Effect of eltrombopag on platelet counts and bleeding during treatment of chronic idiopathic thrombocytopenic purpura: a randomised, double-blind, placebo-controlled trial. Lancet. 373:641–8. DOI: 10.1016/S0140-6736(09)60402-5. PMID: 19231632.

63. Shirasugi Y, Ando K, Miyazaki K, et al. 2011; Romiplostim for the treatment of chronic immune thrombocytopenia in adult Japanese patients: a double-blind, randomized phase III clinical trial. Int J Hematol. 94:71–80. DOI: 10.1007/s12185-011-0886-8. PMID: 21706145.

64. Tomiyama Y, Miyakawa Y, Okamoto S, et al. 2012; A lower starting dose of eltrombopag is efficacious in Japanese patients with previously treated chronic immune thrombocytopenia. J Thromb Haemost. 10:799–806. DOI: 10.1111/j.1538-7836.2012.04695.x. PMID: 22409309.

65. Yang R, Li J, Jin J, et al. 2017; Multicentre, randomised phase III study of the efficacy and safety of eltrombopag in Chinese patients with chronic immune thrombocytopenia. Br J Haematol. 176:101–10. DOI: 10.1111/bjh.14380. PMID: 27734464.

66. Kuter DJ, Rummel M, Boccia R, et al. 2010; Romiplostim or standard of care in patients with immune thrombocytopenia. N Engl J Med. 363:1889–99. DOI: 10.1056/NEJMoa1002625. PMID: 21067381.

67. Provan D, Arnold DM, Bussel JB, et al. 2019; Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv. 3:3780–817. DOI: 10.1182/bloodadvances.2019000812. PMID: 31770441. PMCID: PMC6880896.

68. Blanchette VS, Luke B, Andrew M, et al. 1993; A prospective, randomized trial of high-dose intravenous immune globulin G therapy, oral prednisone therapy, and no therapy in childhood acute immune thrombocytopenic purpura. J Pediatr. 123:989–95. DOI: 10.1016/S0022-3476(05)80400-7. PMID: 8229536.

69. Sartorius JA. 1984; Steroid treatment of idiopathic thrombocytopenic purpura in children. Preliminary results of a randomized cooperative study. Am J Pediatr Hematol Oncol. 6:165–9. DOI: 10.1097/00043426-198406020-00008. PMID: 6540532.
70. Fujisawa K, Iyori H, Ohkawa H, et al. 2000; A prospective, randomized trial of conventional, dose-accelerated corticosteroids and intravenous immunoglobulin in children with newly diagnosed idiopathic thrombocytopenic purpura. Int J Hematol. 72:376–83. PMID: 11185998.
71. Blanchette V, Imbach P, Andrew M, et al. 1994; Randomised trial of intravenous immunoglobulin G, intravenous anti-D, and oral prednisone in childhood acute immune thrombocytopenic purpura. Lancet. 344:703–7. DOI: 10.1016/S0140-6736(94)92205-5. PMID: 7915773.

72. Imbach P, Wagner HP, Berchtold W, et al. 1985; Intravenous immunoglobulin versus oral corticosteroids in acute immune thrombocytopenic purpura in childhood. Lancet. 2:464–8. DOI: 10.1016/S0140-6736(85)90400-3. PMID: 2863492.

73. Celik M, Bulbul A, Aydogan G, et al. 2013; Comparison of anti-D immunoglobulin, methylprednisolone, or intravenous immunoglobulin therapy in newly diagnosed pediatric immune thrombocytopenic purpura. J Thromb Thrombolysis. 35:228–33. DOI: 10.1007/s11239-012-0801-z. PMID: 22956408.

74. Bussel JB, Buchanan GR, Nugent DJ, et al. 2011; A randomized, double-blind study of romiplostim to determine its safety and efficacy in children with immune thrombocytopenia. Blood. 118:28–36. DOI: 10.1182/blood-2010-10-313908. PMID: 21502541.

75. Grainger JD, Locatelli F, Chotsampancharoen T, et al. 2015; Eltrombopag for children with chronic immune thrombocytopenia (PETIT2): a randomised, multicentre, placebo-controlled trial. Lancet. 386:1649–58. DOI: 10.1016/S0140-6736(15)61107-2. PMID: 26231455.

76. Elalfy MS, Abdelmaksoud AA, Eltonbary KY. 2011; Romiplostim in children with chronic refractory ITP: randomized placebo controlled study. Ann Hematol. 90:1341–4. DOI: 10.1007/s00277-011-1172-9. PMID: 21318572.

77. González-López TJ, Pascual C, Álvarez-Román MT, et al. 2015; Successful discontinuation of eltrombopag after complete remission in patients with primary immune thrombocytopenia. Am J Hematol. 90:E40–3. DOI: 10.1002/ajh.23900. PMID: 25400215.

78. Iino M, Sakamoto Y, Sato T. 2020; Treatment-free remission after thrombopoietin receptor agonist discontinuation in patients with newly diagnosed immune thrombocytopenia: an observational retrospective analysis in real-world clinical practice. Int J Hematol. 112:159–68. DOI: 10.1007/s12185-020-02893-y. PMID: 32476083.

79. Newland A, Godeau B, Priego V, et al. 2016; Remission and platelet responses with romiplostim in primary immune thrombocytopenia: final results from a phase 2 study. Br J Haematol. 172:262–73. DOI: 10.1111/bjh.13827. PMID: 26537623.

80. Ghanima W, Khelif A, Waage A, et al. 2015; Rituximab as second-line treatment for adult immune thrombocytopenia (the RITP trial): a multicentre, randomized, double-blind, placebo-controlled trial. Lancet. 385:1653–61. DOI: 10.1016/S0140-6736(14)61495-1.
81. Poudyal BS, Sapkota B, Shrestha GS, Thapalia S, Gyawali B, Tuladhar S. 2016; Safety and efficacy of azathioprine as a second line therapy for primary immune thrombocytopenic purpura. JNMA J Nepal Med Assoc. 55:16–21. DOI: 10.31729/jnma.2832. PMID: 27935917.

82. Verlin M, Laros RK Jr, Penner JA. 1976; Treatment of refractory thrombocytopenic purpura with cyclophosphamine. Am J Hematol. 1:97–104. DOI: 10.1002/ajh.2830010111. PMID: 988746.
83. Choudhary DR, Naithani R, Mahapatra M, Kumar R, Mishra P, Saxena R. 2008; Efficacy of cyclosporine as a single agent therapy in chronic idiopathic thrombocytopenic purpura. Haematologica. 93:e61–2. discussion e63DOI: 10.3324/haematol.13481. PMID: 18827257.

84. Kim SW, Rice L, McCarthy JJ. 1997; Efficacy of danazol with autoimmune thrombocytopenia. Clin Appl Thromb Hemost. 3:251–5. DOI: 10.1177/107602969700300406.

85. Damodar S, Viswabandya A, George B, Mathews V, Chandy M, Srivastava A. 2005; Dapsone for chronic idiopathic thrombocytopenic purpura in children and adults--a report on 90 patients. Eur J Haematol. 75:328–31. DOI: 10.1111/j.1600-0609.2005.00545.x. PMID: 16146539.

86. Godeau B, Durand JM, Roudot-Thoraval F, et al. 1997; Dapsone for chronic autoimmune thrombocytopenic purpura: a report of 66 cases. Br J Haematol. 97:336–9. DOI: 10.1046/j.1365-2141.1997.412687.x. PMID: 9163598.

87. Patel AP, Patil AS. 2015; Dapsone for immune thrombocytopenic purpura in children and adults. Platelets. 26:164–7. DOI: 10.3109/09537104.2014.886677. PMID: 24512442.

88. Vancine-Califani SM, De Paula EV, Ozelo MC, Orsi FL, Fabri DR, Annichino-Bizzacchi JM. 2008; Efficacy and safety of dapsone as a second-line treatment in non-splenectomized adults with immune thrombocytopenic purpura. Platelets. 19:489–95. DOI: 10.1080/09537100802315110. PMID: 18979360.

89. Zaja F, Marin L, Chiozzotto M, Puglisi S, Volpetti S, Fanin R. 2012; Dapsone salvage therapy for adult patients with immune thrombocytopenia relapsed or refractory to steroid and rituximab. Am J Hematol. 87:321–3. DOI: 10.1002/ajh.22266. PMID: 22190262.

90. Hou M, Peng J, Shi Y, et al. 2003; Mycophenolate mofetil (MMF) for the treatment of steroid-resistant idiopathic thrombocytopenic purpura. Eur J Haematol. 70:353–7. DOI: 10.1034/j.1600-0609.2003.00076.x. PMID: 12756016.

91. Miano M, Ramenghi U, Russo G, et al. 2016; Mycophenolate mofetil for the treatment of children with immune thrombocytopenia and Evans syndrome. A retrospective data review from the Italian association of paediatric haematology/oncology. Br J Haematol. 175:490–5. DOI: 10.1111/bjh.14261. PMID: 27447678.

92. Taylor A, Neave L, Solanki S, et al. 2015; Mycophenolate mofetil therapy for severe immune thrombocytopenia. Br J Haematol. 171:625–30. DOI: 10.1111/bjh.13622. PMID: 26250874.

93. Ahn YS, Harrington WJ, Seelman RC, Eytel CS. 1974; Vincristine therapy of idiopathic and secondary thrombocytopenias. N Engl J Med. 291:376–80. DOI: 10.1056/NEJM197408222910802. PMID: 4847353.

94. Fresneau B, Petit A, Courcoux MF, et al. 2011; Vinblastine in the treatment of children and adolescents with refractory immune thrombocytopenia. Am J Hematol. 86:785–7. DOI: 10.1002/ajh.22081. PMID: 21800354.

95. Park YH, Yi HG, Lee MH, Kim CS, Lim JH. 2016; Clinical efficacy and tolerability of vincristine in splenectomized patients with refractory or relapsed immune thrombocytopenia: a retrospective single-center study. Int J Hematol. 103:180–8. DOI: 10.1007/s12185-015-1903-0. PMID: 26588926.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download