Since its outbreak in December 2019 at Wuhan [1], the severe acute respiratory syndrome coronavirus-2 is prevalent worldwide, despite the advent of highly effective vaccines in November 2020 [2]. The world is reeling in the pandemic with slow herd immunity and viral mutants, mostly owing to the absolute shortage of vaccine rollout or unequal distribution with no exception in Korea. During mass vaccination, unexpected rare adverse effects of the vaccine surfaced. Thrombotic thrombo-cytopenic syndrome (TTS) with clinical features resembling heparin-induced thrombocytopenia (HIT) has been a concern, although the incidence was less than 10 per million estimated exposures in the United Kingdom and European Union. The first case of TTS related to coronavirus disease 2019 (COVID-19) vaccine in Korea was seen in a 30-year-old man who received AstraZenaca vaccine in April 2021. The second case in a 33-year-old man who received Johnson and Johnson vaccine in May 2021 was fatal [3]. Another case of vaccine-related death of a young Korean man in his twenties was reported to be caused by myocarditis after receiving the Pfizer-BioNTech messenger ribonucleic acid (mRNA) vaccine in June 2021.
We report a case of TTS in an 85-year-old woman who received mRNA COVID-19 vaccine (Pfizer-BioNTech) on day -18 with no immediate event. Weakness, dysarthria and walking difficulty were noted on day -3 followed by myalgia, when she was brought to the emergency room on day 1. Severe thrombocytopenia (4,000/mL) was found on the day refractory to platelet concentrates transfusion. Her past medical history showed hypertension and atrial fibrillation placed on hydrochlorothiazide 15 mg, amlodipine 5 mg, cande-sartan 16 mg, and carvedilol 8 mg, hyper-cholesterolemia placed on atorvastatin 10 mg, and depressive illness placed on mirtazapine 7.5 mg (all once a day). She was never exposed to heparin. History of neuromuscular disease was denied. Examination disclosed an obese and alert woman based on the World Health Organization performance IV for weakness of the lower limbs. Fever, pallor, jaundice, lymphadenopathy, or bleeding tendency was absent. The heart was irregular in rhythm with no murmur. Motor power of the limbs was grade 3/5 with intact sensory but without focal neurological deficit except for slurred speech. The remainder of the exam was negative. Table 1 shows laboratory test results.
Chest radiograph, chest computed tomography (CT), and abdominal CT were unremarkable except for cardiomegaly with C/T ratio of 0.6. Brain CT or MRI was not conducted. The effect of platelet concentrates transfusion of 18 units over days 2–4 was very short. Under the impression of vaccine-induced immune thrombocytopenia (ITP), myositis, and possible Guillain-Barré syndrome (GBS), high dose immunoglobulin (IVIG) was administered at 1 g/kg on days 2 and 4, which caused a dramatic rise in platelet count (Fig. 1A) with drop in D-dimer (Fig. 1B) and CK levels (Fig. 1C), and the disappearance of dysarthria. Nerve conduction studies performed in the median, ulnar, proximal peroneal, tibial, and sural nerves on day 23 were un-remarkable. Spinal tap and electromyelogram were not performed for thrombocytopenia. Muscle biopsy was not performed. Chest CT performed after an episode of dyspnea on day 31 revealed pulmonary embolism (Fig. 2), and apixaban 10 mg twice a day was started on day 32. Antibody to platelet factor-4 (PF4)-heparin complex by enzyme-linked immunosorbent assay, tested using the Immucor Lifecodes PF4 IgG assay kit, was positive (0.71 U/mL, cut off value 0.4) on day 36. The Acustar HIT test for PF4-heparin complex by automated quantitative chemiluminescent immunoassay was negative (0.6 U/mL, cutoff value 1.0). The resolution of embolism was documented using CT on day 38. Muscle weakness improved (motor power grade 4/5), and she could be ambulated on day 90 as of this writing.
Thrombocytopenia is a rare complication among recipients of currently available COVID-19 vaccination. The viral vector vaccine was known to cause TTS by inducing the PF4 antibody. TTS has been much rarer among recipients of m-RNA than adenovector with some reports of death reports but no data regarding PF4 antibody [4, 5]. TTS with detectable PF4 antibody had not been documented among recipients of m-RNA until July 2021 [6, 7]. Our case presented with thrombocytopenia, myositis/muscle weakness, and pulmonary arterial embolism after receiving m-RNA COVID-19 vaccine, mimicking an immune reaction in multiple organs. IVIG was administered for transfusion refractory ITP, a standard therapy for m-RNA vaccine-induced ITP and GBS. Although GBS has been reported among COVID-19 vaccine recipients regardless of vector, the rarity undiscernible from the incidence of normal population raised controversy doubting the causal relation between GBS and COVID-19 vaccination [8]. In our patient, there was no definite evidence of GBS as shown in the normal nerve conduction studies, making it difficult to conclude that the patient had immune-mediated neuropathy. Defect in sensory, abnormal autonomic nerve function, or ascending paralysis was absent. Acetylcholine receptor antibody was negative. Muscle weakness could be explained by CK elevation. A short period of CK elevation indicated an evident myositis, common among patients with COVID-19 but very uncommon among vaccine recipients [9]. As CK or AST value did not necessarily reflect myositis, sustained muscle weakness with rapid return of muscle enzyme value to normal did not exclude the possibility of myositis. The possibility of rhabdomyolysis caused by 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase was considered, and atorvastatin was stopped while CK value elevated. The possibility of myocarditis was excluded owing to no cardiac dysfunction and near normal value of CK-MB. However, the possibility of GBS could not be excluded given the wide clinical spectrum of GBS and slow recovery of weakness. The second dose of m-RNA vaccine was withheld.
The presence of PF4-heparin complex antibody was unexpected during the workup for the dyspneic episode and pulmonary embolism. Her thrombophilia appeared milder than the fatal case [6] associated with a high titer of PF4-heparin complex antibody. Her atrial fibrillation could be a predisposing factor of pulmonary embolism, PF4-heparin complex antibody has been reported in normal population and a small portion of m-RNA COVID-19 vaccine recipients [10], myositis could be an adverse effect of atorvastatin, and pulmonary embolism and detection of PF4-heparin complex antibody could be coincidental. However, simultaneous appearance of ITP, thrombosis, myositis, and possible GBS should be regarded as more than a coincidence. As the events of adverse effect accumulate, previously held belief that the antibodies to PF4 are exclusively generated by adenoviral vectors is being challenged. The case presented here showed m-RNA COVID-19 vaccine could affect multiple organs. Every rare event after exposure to COVID-19 vaccine in statistical chance similar to natural incidence should be regarded as relevant unless proven otherwise. Elucidation of molecular mimicry in vaccine-induced immune-mediated pathogenesis, difference between typical HIT and vaccine-induced TTP, or the correlation of PF4 antibody titer and spectrum of disease activity could solve the current controversies. Vigilance is needed for every event in every COVID-19 vaccination case. Although m-RNA vaccines were known to be most safe and potent among different vaccines, unexpected adverse effects can lead to death. Whether m-RNA vaccine would be enlisted on the culprit of TTS appears uncertain and needs further study. Vaccine induced ITP and TTS could be the same disease with a different clinical spectrum. Every ITP case in the setting of m-RNA vaccination may require a test for PF4-heparin complex antibody to address that question. Whether m-RNA vaccine could cause myositis, GBS, and affect multiple organs simultaneously, appears uncertain and needs further study.
REFERENCES
1. Zhu N, Zhang D, Wang W, et al. 2020; A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 382:727–33. DOI: 10.1056/NEJMoa2001017. PMID: 31978945. PMCID: PMC7092803.

2. Krause PR, Gruber MF. 2020; Emergency use authorization of covid vaccines-safety and efficacy follow-up considerations. N Engl J Med. 383:e107. DOI: 10.1056/NEJMp2031373. PMID: 33064383.
3. Choi JK, Kim S, Kim SR, et al. 2021; Intracerebral hemorrhage due to thrombosis with thrombocytopenia syndrome after vaccination against COVID-19: the first fatal case in Korea. J Korean Med Sci. 36:e223. DOI: 10.3346/jkms.2021.36.e223. PMID: 34402235. PMCID: PMC8352786.

4. Weintraub K. 2021. Jan. 6. Death of Florida doctor after receiving COVID-19 vaccine under investigation. USA Today.
5. Welsh KJ, Baumblatt J, Chege W, Goud R, Nair N. 2021; Thrombo-cytopenia including immune thrombocytopenia after receipt of mRNA COVID-19 vaccines reported to the Vaccine Adverse Event Reporting System (VAERS). Vaccine. 39:3329–32. DOI: 10.1016/j.vaccine.2021.04.054. PMID: 34006408. PMCID: PMC8086806.

6. Sangli S, Virani A, Cheronis N, et al. 2021; Thrombosis with thrombocytopenia after the messenger RNA-1273 vaccine. Ann Intern Med. 174:1480–2. DOI: 10.7326/L21-0244. PMID: 34181446. PMCID: PMC8251935.

7. Al-Maqbali JS, Al Rasbi S, Kashoub MS, et al. 2021; A 59-year-old woman with extensive deep vein thrombosis and pulmonary thromboembolism 7 days following a first dose of the Pfizer-BioNTech BNT162b2 mRNA COVID-19 vaccine. Am J Case Rep. 22:e932946. DOI: 10.12659/AJCR.932946. PMID: 34117206. PMCID: PMC8212841.

8. Lunn MP, Cornblath DR, Jacobs BC, et al. 2021; COVID-19 vaccine and Guillain-Barré syndrome: let's not leap to associations. Brain. 144:357–60. DOI: 10.1093/brain/awaa444. PMID: 33313690. PMCID: PMC7799242.

9. Mack M, Nichols L, Guerrero DM. 2021; Rhabdomyolysis secondary to COVID-19 vaccination. Cureus. 13:e15004. DOI: 10.7759/cureus.15004. PMID: 34150372. PMCID: PMC8202440.

10. Thiele T, Ulm L, Holtfreter S, et al. 2021; Frequency of positive anti-PF4/polyanion antibody tests after COVID-19 vaccination with ChAdOx1 nCoV-19 and BNT162b2. Blood. 138:299–303. DOI: 10.1182/blood.2021012217. PMID: 33988688. PMCID: PMC8129797.

Fig. 1
Platelet count rise right after intravenous immunoglobulin (IVIG) is administered on 2 and 4 (A). D-dimer drop down right after IVIG is administered (B). Elevated creatine kinase (CK) return to normal (C).
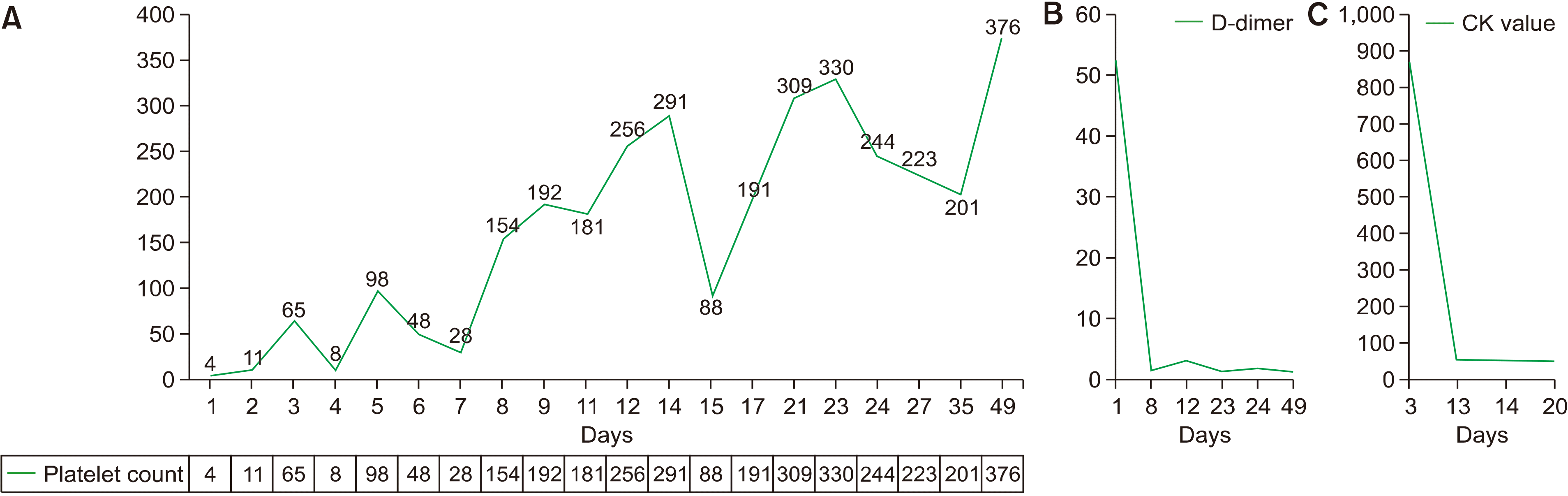
Fig. 2
Focal pulmonary thromboembolism at small branch of the left lower lung basal lateral segment.
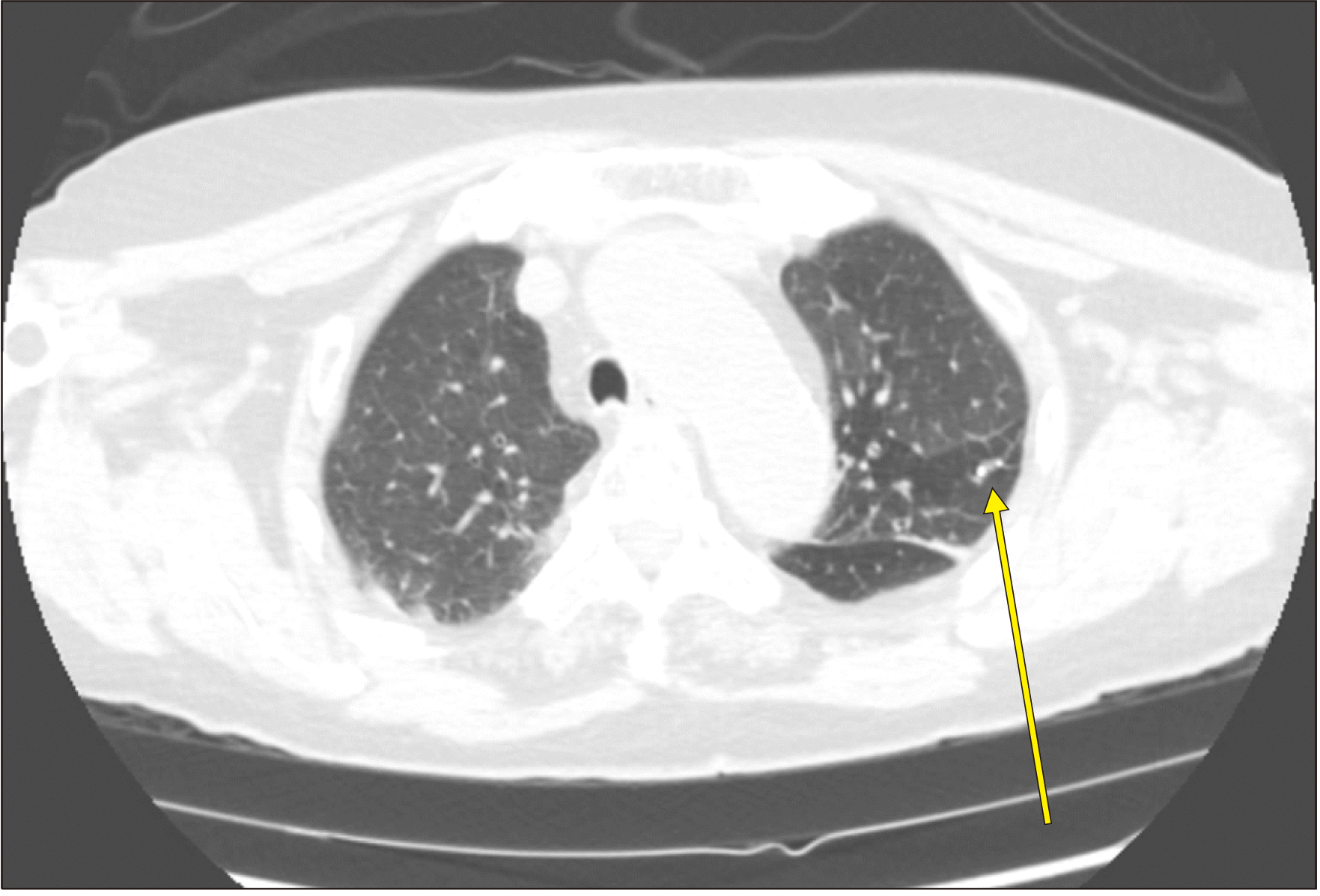
Table 1
Laboratory test results.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download