This article has been
cited by other articles in ScienceCentral.
Abstract
Backgrounds/Aims
Metastatic lesions of the pancreas (PMET) account for 1%–5% of all malignant solid pancreatic lesions (SPL). In this study we evaluated the utility of endoscopic ultrasonography with fine needle aspiration (EUS-FNA) in diagnosing PMET.
Methods
Patients who underwent EUS-FNA at a community referral center between 2011–2017 for SPL were identified. Clinical, radiologic, and EUS-FNA features of those with PMET were compared to those with primary solid tumors of the pancreas: pancreatic adenocarcinoma (PDAC) and neuroendocrine tumors (PNET).
Results
A total of 191 patients were diagnosed with solid pancreatic malignancy using EUS-FNA: 156 PDAC, 27 PNET, and eight (4.2%) had PMET. Patients with PMET were less likely to have abdominal pain (25.0% vs. 76.3% vs. 48.2%; p < 0.01) or obstructive jaundice (37.5% vs. 58.3% vs. 0%; p < 0.01) compared to PDAC and PNET. Those with PMET were more likely to have mass lesions with/without biliary or pancreatic ductal dilatations (100% vs. 86.5% vs. 85.2%; p < 0.01) and lower CA19-9 (82.5 ± 43.21 U/mL vs. 4,639.30 ± 11,489.68 U/mL vs. 10.50 ± 10.89 U/mL; p < 0.01) compared to PDAC and PNET. Endosonographic features were similar among all groups. Seven (87.5%) patients with PMET had a personal history of malignancy prior to PMET diagnosis. The primary malignancy was renal cell carcinoma in five PMET.
Conclusions
PMET are exceedingly rare, comprising less than 5% of SLP. Patients with PMET are less likely to present with symptoms and mostly identified by surveillance imaging for the primary malignancy.
Go to :

Keywords: Endosonography, Biopsy, fine-needle, Neoplasm metastasis, Neuroendocrine tumors, Pancreatic neoplasms
INTRODUCTION
Solid pancreatic lesions (SPL) can be neoplastic or non-neoplastic. Pancreatic duct adenocarcinoma (PDAC) constitutes more than 90% of neoplastic pancreatic lesions, making it the most common primary SPL [
1]. Pancreatic neuroendocrine tumors (PNET) and metastatic lesions to the pancreas (PMET) are less common compared to PDAC, accounting for less than 10% of pancreatic neoplasms [
2]. PMET is the least commonly encountered etiology of neoplastic SPL. Renal cell carcinoma (RCC) is the most common primary cancer to metastasize to the pancreas, followed by lung adenocarcinoma, colorectal cancer, melanoma, ovary cancer, and breast cancer [
3,
4].
In comparison to primary neoplasms of the pancreas, namely PDAC and PNET, PMET has different tumor biology, clinical presentation, and management strategy [
5]. Therefore, accurate diagnosis and identification of PMET are indispensable. Endoscopic ultrasound fine-needle aspiration (EUS-FNA) is a reliable and popular tool for evaluating SPL because of its high sensitivity, specificity, and accuracy [
4]. EUS-FNA has the ability to characterize pancreatic lesions, pancreatic structures (including the pancreatic duct), local invasion, lymph nodes, and other nearby structures. It is also the best modality for obtaining tissue diagnosis for SPLs [
6]. Many studies have demonstrated that EUS-FNA is a reliable and accurate modality for diagnosing PMET [
4,
6]. However, more data are needed to further characterize clinical, cross-sectional, and EUS features distinguishing PMET from PDAC and PNET.
Thus, the aim of this study was to examine demographics, clinical features, biomarker levels, and EUS characteristics of patients diagnosed with PMET by EUS-FNA. PMET was also compared with non-PMET solid neoplasms of the pancreas.
Go to :

PATIENTS AND METHODS
This study performed a single-center retrospective analysis of patients diagnosed with SPLs using EUS-FNA at Sanford USD Medical Center at the University of South Dakota (Sioux Falls, SD, USA). After obtaining approval of the study from our Institutional Review Bboard (no. 00001589), patients who underwent EUS-FNA at our institution between July 15, 2011 and November 30, 2017 for suspected pancreatic lesions were identified. Patients who met the following criteria were included in this study: (1) SPLs on cross-sectional imaging or EUS, (2) diagnosis with PMET, PNET, or PDAC, and (3) available EUS-FNA reports. All patients underwent EUS-FNA examination with a curvilinear echoendoscope. Details regarding demographic data, clinical characteristics at presentation, radiological findings, EUS findings, cytopathology, and clinical course were recorded.
Statistical analysis
SAS 9.4 statistical software (SAS Institute, Cary, NC, USA) was used for all analyses. Descriptive statistics were used to compare demographics, imaging findings, lesion characteristics, and EUS metrics among PMET, PDAC, and PNET. Normal distribution of data was evaluated for continuous variables using Shapiro-Wilk test. If data were normally distributed, a parametric test, Analysis of Variance (ANOVA) was used to compare differences in means for continuous variables. If continuous data were not normally distributed, then a non-parametric test (Kruskal–Wallis test) was used to compare differences in means for continuous variables. Chi-square analysis was used to compare differences in proportions of categorical variables among the three groups. If cells had less than five subjects, then Fisher’s exact test was used to compare categorical data. Statistical significance was accepted at p < 0.05.
Go to :

RESULTS
Baseline characteristics
A total of 191 patients were diagnosed with solid pancreatic malignancy using EUS-FNA in the specified study period at our institution, including 156 PDAC, 27 PNET, and 8 PMET (
Fig. 1). Among those with PMET (n = 8), five (62.5%) had RCC and the remaining three patients had breast adenocarcinoma, breast adenocarcinoma/small cell lung cancer (SCLC), or gastric adenocarcinoma. There were 189 Caucasians (98.9%) and 118 (61.8%) males. For comparison of PMET vs. PDAC vs. PNET, the mean age of presentation was younger in PNET and PMET (68.4 vs. 69.7 vs. 62.9 years;
p = 0.02) and body mass index (BMI) was lower in PMET (25.97 vs. 27.16 vs. 32.21 kg/m
2;
p < 0.01). For comparison of PMET vs. PDAC vs. PNET, those with PDAC were more likely to present with abdominal pain (25.0% vs. 76.3% vs. 48.1%;
p < 0.01) and/or obstructive jaundice (37.5% vs 58.3% vs. 0%;
p < 0.01). Mean carbohydrate antigen (CA19-9) levels were significantly (
p < 0.01) higher in PDAC (4,639.30 ± 11,489.68 U/mL) than in PMET (82.5 ± 43.21 U/mL) and PNET (10.50 ± 10.88 U/mL) (
Table 1).
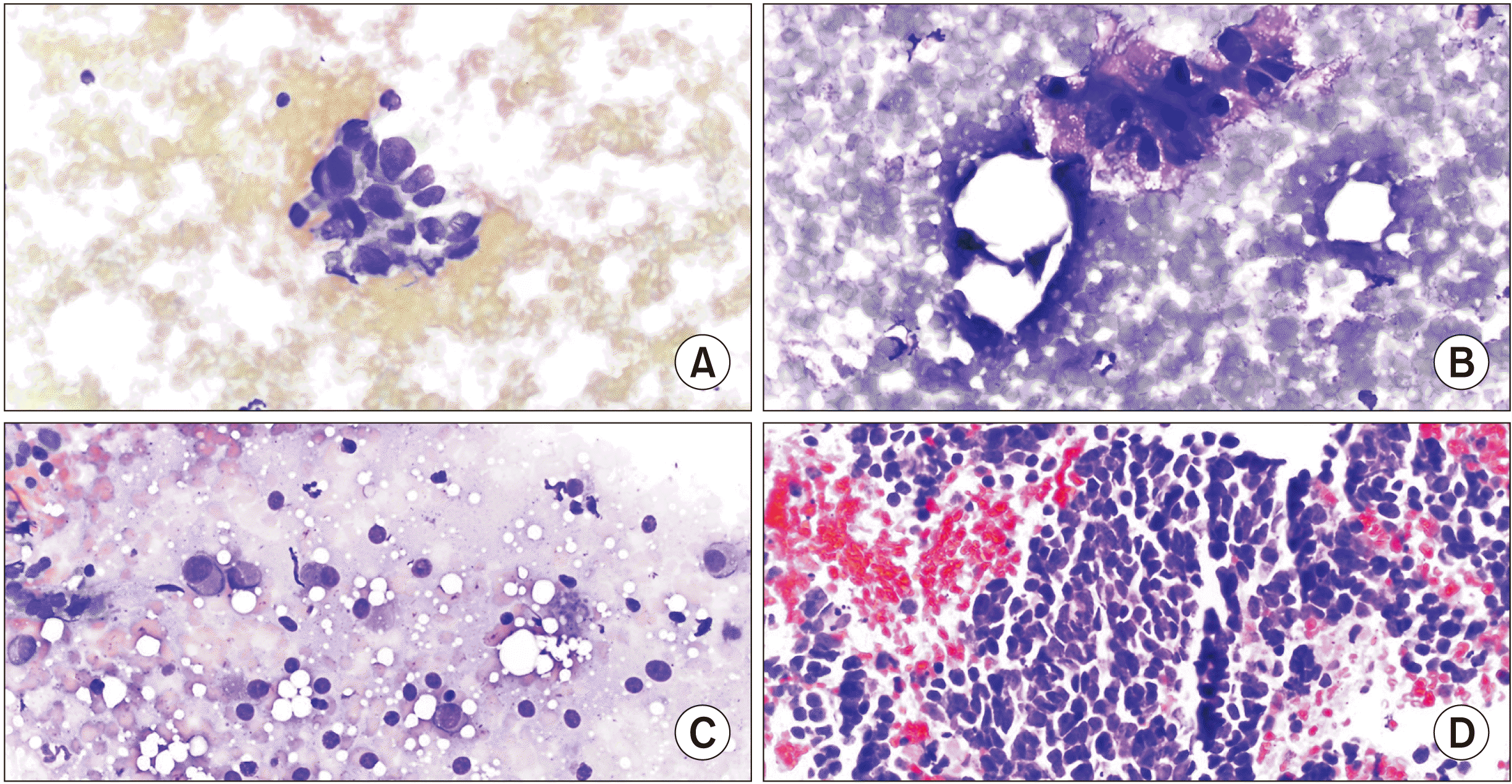 | Fig. 1(A) Pancreatic ductal adenocarcinoma cellular specimen composed of groups of disordered ductal cells having nuclear enlargement, size variation, and hyperchromatic nuclei with prominent nucleoli. (B) Pancreatic neuroendocrine tumor cellular specimen showing loosely cohesive and plasmacytoid cells with finely stippled chromatin and moderate amounts of finely granular cytoplasm. (C) Renal cell carcinoma cellular specimen composed of groups of enlarged nuclei with vacuolated cytoplasm and prominent nucleoli. (D) Small cell lung carcinoma cellular specimen composed of sheets of cells with salt and pepper chromatin, scant cytoplasm, and molding. 
|
Table 1
Comparison of clinical and endoscopic ultrasound features of PMET with PDAC and PNET
|
Characteristic |
PMET (n = 8) |
PDAC (n = 156) |
PNET (n = 27) |
p-value |
|
Age (yr) |
68.38 ± 10.56 |
69.65 ± 10.79 |
62.85 ± 16.62 |
0.02a)
|
|
Male sex |
4 (50.0) |
98 (62.8) |
16 (59.3) |
0.71 |
|
Caucasian ethnicity |
8 (100) |
154 (98.7) |
27 (100) |
1.00 |
|
CA19-9 (U/mL) |
82.5 ± 43.21 (n = 4) |
4,639 ±11,489.68 (n = 132) |
10.50 ± 10.89 (n = 8) |
< 0.01a)
|
|
BMI (kg/m2) |
25.97 ± 5.55 |
27.16 ± 5.77 |
32.21 ± 6.79 |
< 0.01a)
|
|
Abdominal pain |
2 (25.0) |
119 (76.3) |
13 (48.2) |
< 0.01 |
|
Obstructive Jaundice |
3 (37.5) |
91 (58.3) |
0 (0) |
< 0.01 |

Characteristics of cross-sectional imaging
The initial cross-sectional imaging was negative for malignancy in four (2.6%) patients with PDAC and in three (11.5%) patients with PNET. In comparison of PMET vs. PDAC vs. PNET, mean diameters of mass lesions on cross sectional imaging (computerized tomography [CT], magnetic resonance imaging [MRI], or ultrasonography [US]) at the time of diagnosis were similar among the three groups (31.88 vs. 32.72 vs. 27.81 mm,
p = 0.26) (
Table 2). Mass lesions with/without pancreatic or biliary ductal strictures/dilatations were more common in PMET (100% vs. 86.6% vs. 85.2%;
p < 0.01), whereas biliary or pancreatic ductal strictures/dilatations alone was more prevalent in PDAC (0% vs. 10.9% vs. 3.7%;
p < 0.01). Most mass lesions were in the head/neck of pancreas in those with PMET and PDAC (37.5% vs. 59.6% vs. 22.2%) and in the tail of pancreas in those with PNET (25.0% vs. 10.3% vs. 48.2%;
p < 0.01) (
Table 2).
Table 2
Cross sectional imaging in patients with PMETs, PDAC, or PNETs
|
Characteristic |
PMET (n = 8) |
PDAC (n = 156) |
PNET (n = 27) |
p-value |
|
Mean diameter of SPL (mm) |
31.88 ± 25.85 |
33.72 ± 13.47 |
27.80 ± 19.78 |
0.26b)
|
|
Imaging findings |
|
|
|
< 0.01 |
|
Negative |
0 |
4 (2.6) |
3 (11.1) |
|
|
Ductal stricture/dilatationa)
|
0 |
17 (10.9) |
1 (3.7) |
|
|
Mass lesion alone |
4 (50.0) |
60 (38.5) |
22 (81.5) |
|
|
Stricture/dilatation + mass lesion |
4 (50.0) |
75 (48.1) |
1 (3.7) |
|
|
Lesion location on imaging |
|
|
|
< 0.01 |
|
Head/neck |
3 (37.5) |
93 (59.6) |
6 (22.2) |
|
|
Body |
2 (25.0) |
19 (12.2) |
4 (14.8) |
|
|
Tail |
2 (25.0) |
16 (10.3) |
13 (48.2) |
|
|
Unspecified |
1 (12.5) |
17 (10.9) |
1 (3.7) |
|
|
None |
0 (0) |
11 (7.1) |
3 (11.1) |
|

Characteristics of endoscopic ultrasonography
The initial EUS-FNA cytology was negative for malignancy in 12.5% of PMET, 1.9% of PDAC, and 7.4% of PNET. It was positive for malignancy in 62.5% of PMET, 91.7% of PDAC, and 88.9% of PNET. In addition, 25.0% of PMET showed atypical cytology compared to 2.6% of PDAC and 3.7% of PNET (
p = 0.02). Locations of mass lesions on EUS correlated with mass location on cross sectional imaging are shown in
Table 2. Most lesions were in the head/neck of the pancreas in PDAC and in the tail of pancreas in PNET (
Table 3). In PMET, the location of the lesion was not provided in about 50% of patients’ EUS-FNA reports. A total of 37.5% of lesions were located in the head/neck of pancreas. Six (75.0%) patients had a solitary mass on EUS. One patient had two masses and one patient had four masses. Previous biliary/pancreatic stents, diagnosing chronic pancreatitis with EUS, presence of onsite cytopathologist, core biopsy during EUS, and the need for more than one EUS-FNA were similar among the three groups (PMET, PDAC, and PNET) (
Table 3).
Table 3
Comparison of EUS characteristics among PMETs, PDAC, and PNETs
|
Characteristic |
PMET (n = 8) |
PDAC (n = 156) |
PNET (n = 27) |
p-value |
|
EUS mass lesion location |
|
|
|
< 0.01 |
|
Head/neck |
3 (37.5) |
105 (67.3) |
5 (18.5) |
|
|
Body |
1 (12.5) |
24 (15.4) |
7 (25.9) |
|
|
Tail |
0 (0) |
18 (11.5) |
13 (48.2) |
|
|
Other/unspecified |
4 (50.0) |
7 (4.5) |
2 (7.4) |
|
|
None |
0 (0) |
2 (1.3) |
0 (0) |
|
|
EUS-FNA cytology |
|
|
|
0.02 |
|
Negative |
1 (12.5) |
3 (1.9) |
2 (7.4) |
|
|
Atypical |
2 (25.0) |
4 (2.6) |
1 (3.7) |
|
|
Suspicious for malignancy |
0 (0) |
6 (3.9) |
0 (0) |
|
|
Positive |
5 (62.5) |
143 (91.7) |
24 (88.9) |
|
|
Previous stents |
|
|
|
0.17 |
|
Pancreatic stents |
1 (12.5) |
6 (3.9) |
0 (0) |
|
|
Biliary stents |
1 (12.5) |
7 (4.5) |
0 (0) |
|
|
None |
6 (75.0) |
143 (91.7) |
27 (100) |
|
|
CP per Rosemont criteria |
|
|
|
1.00 |
|
Negative |
7 (87.5) |
135 (86.5) |
24 (88.9) |
|
|
Positive |
1 (12.5) |
21 (13.5) |
3 (11.1) |
|
|
On site cytopathologist |
|
|
|
0.86 |
|
Yes |
8 (100) |
143 (91.7) |
24 (88.9) |
|
|
No |
0 (0) |
13 (8.3) |
3 (11.1) |
|
|
Core biopsy during EUS |
|
|
|
0.69 |
|
Yes |
0 (0) |
6 (3.9) |
0 (0) |
|
|
No |
8 (100) |
150 (96.2) |
27 (100) |
|
|
More than one EUS |
|
|
|
0.76 |
|
No |
7 (87.5) |
141 (91.0) |
25 (92.6) |
|
|
Yes |
1 (12.5) |
14 (9.0)a)
|
2 (7.4) |
|

Characteristics and outcomes of patients with PMET
Table 4 outlines each individual patient’s demographics, previous cancer diagnosis, and time from primary cancer diagnosis to PMET cancer diagnosis, and outcomes of patients with PMET. Seven (87.5%) patients did not have evidence of extra-pancreatic mass or any other metastatic lesions at the time of referral for EUS. One patient had an adrenal nodule suspicious for metastasis in addition to a cerebellum mass. EUS-FNA was considered to obtain tissues for suspected malignancy. CA19-9 was elevated in the three patients with PMET who had available results (58.0 U/mL in a breast adenocarcinoma patient, 147.0 U/mL in a gastric adenocarcinoma patient, and 67.0 U/mL in a breast adenocarcinoma/SCLC patient). The primary cancer diagnosis in five (62.5%) patients was RCC (
Fig. 1C). In the remaining three patients, primary cancer diagnosis was breast adenocarcinoma, gastric adenocarcinoma, or SCLC. For one patient, the primary cancer could not be ascertained from cytology. This patient had a history of both breast adenocarcinoma and SCLC (
Fig. 1D). Seven (87.5%) out of eight patients had a known personal history of cancer prior to development of PMET. Three (37.5%) patients were diagnosed with PMET through active surveillance for RCC. Time from primary cancer diagnosis until PMET was variable among patients (range, 3–168 months). One patient, a 71-year-old female with a prior history of breast adenocarcinoma diagnosed 72 months prior to presentation with jaundice, pruritus, and biliary/pancreatic ductal dilatation on CT abdominal imaging and treated with radical mastectomy, chemotherapy, and hormonal therapy had an initial EUS-FNA that was negative for malignancy. Given the high suspicion of malignancy, a repeat EUS-FNA performed two weeks later was positive for malignancy. Only one patient with PMET underwent surgical resection (total pancreatectomy of PMET for RCC). Five (62.5%) patients were treated with chemotherapy with or without immunotherapy/chemotherapy based on the primary cancer diagnosis. Two patients did not receive treatment for PMET. They opted for palliative care. Four (50.0%) patients died within 4–28 months of PMET diagnosis. Two (25.0%) patients were alive at 21–34 months after PMET diagnosis. One patient who underwent total pancreatectomy was alive at 81 months after PMET diagnosis. One patient was lost to follow up (
Table 4). In all PMETs, 25-gaguge needles were used. A 22-gauge needle was only used in one patient (a 71-year-old female with a prior history of breast adenocarcinoma and an initial negative EUS-FNA). The average number of needle passes using 25-gauge was 2.6 ± 1.6. It was one pass for the 22-gauge needle after five passes using a 25-gauge needle.
Table 4
Clinical characteristics of patients with metastatic lesions of the pancreas (PMET)
|
Patient |
Age (yr)/sex |
History of cancer |
CA19-9 (U/mL)a)
|
Mass diameter (mm) |
Interval to PMET diagnosis (mon)b)
|
Method of diagnosis |
Method of treatment |
Outcome/follow up (mon) |
Final diagnosis |
|
1 |
71/F |
Yes |
58.0 |
14.0 |
72 |
Ductal dilatation |
Chemotherapy, hormonal Tx |
Alive/34 |
Breast adenocarcinoma |
|
2 |
85/F |
None |
147.0 |
91.0 |
3 |
Mass on imaging |
No Tx |
Death/4 |
Gastric adenocarcinoma |
|
3 |
66/F |
Yes |
67.0 |
32.0 |
18 |
Mass on imaging |
Chemotherapy |
Death/13 |
Breast adenocarcinoma/SCLC |
|
4 |
65/M |
Yes |
None |
29.0 |
168 |
Active surveillance |
Chemotherapy, immunotherapy |
Death/28 |
RCC |
|
5 |
79/M |
Yes |
None |
41.0 |
77 |
Mass on imaging |
Chemotherapy |
Death/26 |
RCC |
|
6 |
54/M |
Yes |
None |
19.0 |
84 |
Active surveillance |
Chemotherapy |
Alive/21 |
RCC |
|
7 |
56/M |
Yes |
None |
15.0 |
102 |
Active surveillance |
No Tx |
Lost to follow up/2 |
RCC |
|
8 |
49/F |
Yes |
None |
12.0 |
150 |
Mass on imaging |
Total pancreatectomy |
Alive/81 |
RCC |

Go to :

DISCUSSION
This report highlights the fact that PMET can present as solitary pancreatic masses. Although previous reports have highlighted this issue, most of these reports originated from large academic and tertiary referral centers. This study details these lesions from a community referral center. Given the very rare incidence of PMET (1%–5% of all pancreatic malignancies), diagnosis requires a high index of suspicion [
7,
8]. Evaluating PMET is challenging because of the rarity of PMET (4.2% of all solid pancreatic malignancies in our cohort), the absence of symptoms of biliary obstruction or abdominal pain on clinical presentation in comparison to non-PMETs neoplasms, and the similarity of FNA-cytology between RCC (the most common cellular type of PMET) and PDAC [
2,
3,
6]. Therefore, it is important to take into account cytological features and immunohistochemistry in the backdrop of pertinent medical history. Immunohistochemistry is not routinely performed for pancreatic mass lesions. Thus, it is important to have a high index of suspicion for PMET at the time of FNA to collect additional samples for immunohistochemistry. Main findings of this study are 1) patients with PMET are less likely to present with abdominal pain or jaundice than those with PDAC and PNET, 2) EUS-FNA is less sensitive in PMET than in PDAC and PNET, 3) high degree of suspicion for PMET is needed, especially for patients with a prior history of malignancy.
Results of our study demonstrated that patients with PMET were younger than those with PDAC but older than those with PNET, consistent with prior studies [
4,
9]. In addition, the absence of biliary obstruction or abdominal pain symptoms in patients with PMET was also in concordance with studies that compared PMET with PDAC and PNET [
4,
6]. Regarding neoplasm location, pancreatic head/neck was the most common location for metastasis in this study and other studies [
4,
10]. RCC is the most common primary tumor in PMET reported in literature, although some studies have described that lung cancer is the most common one [
4,
7,
8,
11-
13]. This might be due to the tendency for hematogenous spread in RCC [
14]. Nevertheless, metastases (especially from RCC) mimics primary pancreatic carcinomas (mainly PDAC) both clinically and cytologically. Clinical, radiological, and immune-histochemical examinations are extremely important when evaluating SPL [
10]. Patients with PMET are often identified on cancer surveillance using cross-sectional imaging in patients with a history of a remote malignancy who are in clinical remission or in patients with an active malignancy [
15]. However, malignancies presenting as metastases to the pancreas have been described [
16]. In our study, rapid on-site evaluation significantly enhanced our diagnostic yield and helped us avert any diagnostic dilemma.
Evaluation of patients with current or remote history of malignancy presenting with pancreatic masses can be challenging. Differentiating PMET from
de novo adenocarcinoma is of paramount importance in these patients [
4]. While adenocarcinoma of the pancreas is diagnosed in most patients with SPL, it is crucial to consider PMET in these patients. In our PMET patients’ cohort, seven (87.5%) out of eight patients had a past medical history of malignancy. Diagnosis of PMET in our study was done through cancer surveillance in patients with a recent history of malignancy or through abdominal imaging performed for suggestive symptoms in patients with a history of recent/remote malignancy.
Many studies have reported that the sensitivity, specificity, positive predictive value, and accuracy of EUS-FNA for diagnosing PMET are 77%–85%, 98%–100%, 98%–100%, and 80%–98%, respectively [
4,
10]. In addition, EUS-FNA for SPL is a relatively safe procedure with low incidence of local, systemic, or major complications. In a study of 134 patients who had EUS-FNA for SPL, no complication was observed [
17]. We did not observe any complications related to EUS-FNA in our patient cohort either.
This study has many limitations. First, this study had a small sample size due to the rarity of PMET, which hindered the statistical power of this study. The retrospective design of this study made it more susceptible to selective bias. Given the rare nature of PMET, larger multi-center prospective studies are needed to characterize PMET in the future.
In our sutdy, we demonstrate that PMETs are uncommon, accounting for less than 5% of all pancreatic malignancies. RCC is the most common primary malignancy that metastasizes to the pancreas. EUS-FNA is a reliable and accurate tool for evaluating patients with high pre-test probability of PMETs.
Go to :

ACKNOWLEDGEMENTS
This manuscript was presented as an abstract at the American Journal of Gastroenterology meeting in San Antonio, Texas, USA in October 2019 (abstract number 2791, volume 114).
Go to :

Notes
Go to :

REFERENCES
1. Feldmann G, Beaty R, Hruban RH, Maitra A. 2007; Molecular genetics of pancreatic intraepithelial neoplasia. J Hepatobiliary Pancreat Surg. 14:224–232. DOI:
10.1007/s00534-006-1166-5. PMID:
17520196. PMCID:
PMC2666331.

2. Gagovic V, Spier BJ, DeLee RJ, Barancin C, Lindstrom M, Einstein M, et al. 2012; Endoscopic ultrasound fine-needle aspiration characteristics of primary adenocarcinoma versus other malignant neoplasms of the pancreas. Can J Gastroenterol. 26:691–696. DOI:
10.1155/2012/761721. PMID:
23061060. PMCID:
PMC3472907.

3. Alzahrani MA, Schmulewitz N, Grewal S, Lucas FV, Turner KO, McKenzie JT, et al. 2012; Metastases to the pancreas: the experience of a high volume center and a review of the literature. J Surg Oncol. 105:156–161. DOI:
10.1002/jso.22009. PMID:
21725976.

4. Krishna SG, Bhattacharya A, Ross WA, Ladha H, Porter K, Bhutani MS, et al. 2015; Pretest prediction and diagnosis of metastatic lesions to the pancreas by endoscopic ultrasound-guided fine needle aspiration. J Gastroenterol Hepatol. 30:1552–1560. DOI:
10.1111/jgh.12973. PMID:
25867963.

5. Sperti C, Pozza G, Brazzale AR, Buratin A, Moletta L, Beltrame V, et al. 2016; Metastatic tumors to the pancreas: a systematic review and meta-analysis. Minerva Chir. 71:337–344. PMID:
27412234.
6. Atiq M, Bhutani MS, Ross WA, Raju GS, Gong Y, Tamm EP, et al. 2013; Role of endoscopic ultrasonography in evaluation of metastatic lesions to the pancreas: a tertiary cancer center experience. Pancreas. 42:516–523. DOI:
10.1097/MPA.0b013e31826c276d. PMID:
23211369.

7. Layfield LJ, Hirschowitz SL, Adler DG. 2012; Metastatic disease to the pancreas documented by endoscopic ultrasound guided fine-needle aspiration: a seven-year experience. Diagn Cytopathol. 40:228–233. DOI:
10.1002/dc.21564. PMID:
22334524.

8. Adsay NV, Andea A, Basturk O, Kilinc N, Nassar H, Cheng JD. 2004; Secondary tumors of the pancreas: an analysis of a surgical and autopsy database and review of the literature. Virchows Arch. 444:527–535. DOI:
10.1007/s00428-004-0987-3. PMID:
15057558.

9. Turner BG, Cizginer S, Agarwal D, Yang J, Pitman MB, Brugge WR. 2010; Diagnosis of pancreatic neoplasia with EUS and FNA: a report of accuracy. Gastrointest Endosc. 71:91–98. DOI:
10.1016/j.gie.2009.06.017. PMID:
19846087.

10. Waters L, Si Q, Caraway N, Mody D, Staerkel G, Sneige N. 2014; Secondary tumors of the pancreas diagnosed by endoscopic ultrasound-guided fine-needle aspiration: a 10-year experience. Diagn Cytopathol. 42:738–743. DOI:
10.1002/dc.23114. PMID:
24554612.

11. Smith AL, Odronic SI, Springer BS, Reynolds JP. 2015; Solid tumor metastases to the pancreas diagnosed by FNA: a single-institution experience and review of the literature. Cancer Cytopathol. 123:347–355. DOI:
10.1002/cncy.21541. PMID:
25828394.

12. Raymond SLT, Yugawa D, Chang KHF, Ena B, Tauchi-Nishi PS. 2017; Metastatic neoplasms to the pancreas diagnosed by fine-needle aspiration/biopsy cytology: a 15-year retrospective analysis. Diagn Cytopathol. 45:771–783. DOI:
10.1002/dc.23752. PMID:
28603895.

13. Olson MT, Wakely PE Jr, Ali SZ. 2013; Metastases to the pancreas diagnosed by fine-needle aspiration. Acta Cytol. 57:473–480. DOI:
10.1159/000352006. PMID:
24021904.

14. Sellner F, Tykalsky N, De Santis M, Pont J, Klimpfinger M. 2006; Solitary and multiple isolated metastases of clear cell renal carcinoma to the pancreas: an indication for pancreatic surgery. Ann Surg Oncol. 13:75–85. DOI:
10.1245/ASO.2006.03.064. PMID:
16372157.

15. El Hajj II, LeBlanc JK, Sherman S, Al-Haddad MA, Cote GA, McHenry L, et al. 2013; Endoscopic ultrasound-guided biopsy of pancreatic metastases: a large single-center experience. Pancreas. 42:524–530. DOI:
10.1097/MPA.0b013e31826b3acf. PMID:
23146924.
16. Hult M, Sandstrøm H, Wittendorff HE. 2015; [Prostate cancer presenting with diffuse infiltration of metastases to the pancreas]. Ugeskr Laeger. 177:V09140491. Danish. PMID:
25650518.
17. O'Toole D, Palazzo L, Arotçarena R, Dancour A, Aubert A, Hammel P, et al. 2001; Assessment of complications of EUS-guided fine-needle aspiration. Gastrointest Endosc. 53:470–474. DOI:
10.1067/mge.2001.112839. PMID:
11275888.
Go to :






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download