Abstract
Backgrounds/Aims
Postoperative pancreatic fistula (POPF) after pancreaticoduodenectomy (PD) remains a dreadful complication. Duct-to-mucosa pancreaticojejunostomy (DTMPJ) is a commonly performed anastomosis after PD. This study aims to evaluate whether there is a size limit of pancreatic duct below which POPF rate increases significantly after DTMPJ.
Methods
A retrospective study was performed from a database with prospectively collected data on consecutive patients undergoing DTMPJ.
Results
Between the years 2003 and 2019, a total of 288 patients with DTMPJ were recruited. POPF occurred in 56.3% of the patients, of which 43.8% were biochemical leak, 8.7% were grade B, and 1.4% were grade C. Overall operative morbidity was 51.4%, of which 19.1% were major complications. Five patients (1.7%) died within 90 days of operation. Patients with grade B/C POPF had significantly soft pancreas (p < 0.001), smaller duct size (p = 0.031), and a diagnosis of carcinoma of the pancreas (p = 0.027). When a clinically significant POPF rate was analysed based on the pancreatic duct diameter, pancreatic duct size ≤ 1 mm had the highest POPF rate (35.7%). There was a significant difference in POPF rate between adjacent ductal diameter ≤ 1 mm and > 1 mm to 2 mm (35.7% vs 13.3%; p = 0.040). Multivariable analysis showed that for the soft pancreas, pancreatic duct diameter ≤ 1 mm was the only significant predictive factor for POPF (p = 0.027).
Pancreaticoduodenectomy (PD) is a commonly indicated operation for malignant or benign pancreatic head and periampullary region pathologies. Though operative mortality of PD has reduced from 12%–14% to 2%–3% in recent decades, postoperative morbidity remains significantly high at approximately 30%–50%, of which postoperative pancreatic fistula (POPF) is the main cause [1-3]. The incidence of POPF is 5% to 30% and it is associated with serious complications like systemic sepsis, organ failure, postpancreatectomy haemorrhage (PPH), and even death [4-7]. So far, various attempts to reduce the occurrence of POPF have been made but no single measure has been shown to be consistently superior.
After PD, the pancreatic remnant is commonly anastomosed to a Roux-en-Y jejunal loop by a pancreaticojejunostomy (PJ). The most commonly preformed PJ is the duct-to-mucosa PJ (DTMPJ), where the pancreatic duct wall is directly sutured to the wall of a small opening in the jejunum [8,9]. Alternatively, a dunking PJ (DPJ) is performed where the whole pancreatic stump is invaginated to the lumen of the jejunum [10,11]. In some cases, pancreaticogastrostomy (PG) is performed, in which instead of joining to jejunum, the pancreatic stump is invaginated to the stomach lumen [12]. Although both PG and DPJ are preferred over DTM anastomosis when the pancreatic duct is very small, studies have shown that there is no significant difference in the rate of POPF between different anastomotic techniques [13-15] and none of the technical variations of PJ or PG anastomosis has been found to be consistently superior to another [16]. Currently, DTMPJ remains the anastomosis of choice after PD in many centers worldwide.
In our center, DTMPJ is the gold standard reconstruction procedure after PD regardless of the size of the pancreatic duct unless the duct opening cannot be recognized on the pancreatic stump or the involved surgeon is not comfortable doing a DTMPJ. For the outer sutures, the pancreatic capsule is sutured to the jejunal serosa using a modified Blumgart’s technique (see below) [17-19]. However, the small pancreatic duct presents a big challenge for DTMPJ; the smaller the pancreatic duct, the more difficulty the anastomosis procedure and the higher the risk of POPF. Traditionally a pancreatic duct size of ≤ 5 mm is regarded as a small duct and a duct size ≤ 3 mm a high-risk factor for POPF [20,21]. So far, there is no safe pancreatic duct size limit for DTMPJ in regard to POPF that has been reported. The objective of this study is to evaluate whether there is a pancreatic duct size below which the POPF rate increases significantly after DTMPJ.
A retrospective study was performed from a database of prospectively collected data on consecutive patients undergoing PD, with special emphasis on POPF in relation to pancreatic duct size. Data collected included patient demographics, operative characteristics, histological diagnosis, and postoperative outcomes. The study was approved by the Joint Chinese University of Hong Kong, New Territories East Cluster Clinical Research Ethics Committee (CREC No. 2020.628).
Between the years 2003 and 2019, a total of 307 consecutive PD were done in our center. Six emergency PD operations that were done following pancreaticoduodenal trauma or bleeding tumors were excluded from the study. Three PD which were hepatopancreaticoduodenectomy were excluded due to ultra- extent of surgery. Ten patients whose pancreatic anastomosis were not fashioned as DTMPJ, including 5 PG and 5 DPJ, were also excluded. Therefore, 288 PD with DTMPJ were analyzed in this study.
Pancreatic duct size was either retrieved from the operative record as measured by the operating surgeon during surgery or by measurement of the pancreatic duct size anterior to the superior mesenteric vein at neck region on preoperative computed tomography (CT) or magnetic resonance imaging (MRI) in magnified view. The pancreatic texture was classified as either soft or hard as determined by the operating surgeon during surgery. POPF was defined and graded according to the 2016 International Study Group for Pancreatic Surgery [4]. Complications were graded according to the Clavien-Dindo classification [22]. Mortality was defined as death within 90 days after the operation [23].
Either a classical Whipple or a pylorus-preserving PD was performed depending on whether an oncological clearance could be achieved. Most operations were carried out using the traditional open approach though a few laparoscopic or robotic PD were attempted. All the operations were done by experienced pancreatic surgeons or trainees under supervision within the same team. In general, a DTMPJ was used for reconstruction of the pancreatic remnant to the gastrointestinal tract unless the pancreatic duct could not be identified or the surgeon was uncomfortable performing a DTMPJ.
A modified Blumgart’s technique was used for anastomosis of the jejunal wall to the pancreatic stump. This technique facilitated the placement of posterior DTM stitches before the approximation of jejunal wall and pancreatic stump, which consisted of one to three (depending on the pancreatic stump size) outer transpancreatic U-sutures using 4/0 polyethylene, with the central one incorporating the pancreatic duct opening. The suture was passed through the whole pancreas from front to back at 1 cm from the cut edge. A seromuscular bite with a horizontal mattress at the jejunum was made as the posterior layer and the same suture passed through the pancreas from back to front to complete the U-suture (Fig. 1A). The opening made on the jejunal wall should be tailored to the size of the pancreatic duct. DTM anastomosis was fashioned with interrupted 5/0 or 6/0 polydioxanone sutures. The posterior row of DTM sutures was tightened and tied after the jejunal wall was approximated to the pancreatic stump by tightening the outer U-sutures (Fig. 1B). The jejunum was moved during the approximation process. Internal pancreatic duct stent was placed selectively. No external pancreatic drainage was used in this study. The anterior rows of DTM sutures were then completed. The transpancreatic U-sutures were completed anteriorly by a seromuscular bite at the jejunum as a horizontal mattress (Fig. 1C, 1D). Prophylactic sandostatin was used in most of the cases. At the end of the operation, one tubal drain was placed in front and another one behind the PJ anastomosis. The drain fluid was collected and checked for amylase content on postoperative day 1, day 3, day 5, and whenever deemed necessary. Drain was removed on postoperative day 4 or 5 if the drain fluid was clear and the drain fluid amylase was low.
Continuous variables are presented in mean (standard deviation), whilst categorical variables are presented in number (percentage). Mann–Whitney U test was used in continuous variables, chi-squared test or Fisher’s exact test was used in categorical variables. To determine the pancreatic duct size which was predictive of the development of POPF, a univariate and multivariable analysis was performed with logistic regression for different pancreatic duct sizes. A p-value of less than 0.05 was considered statistically significant. All statistical analyses were performed by IBM SPSS version 26 (IBM Corp., Armonk, NY, USA).
The patient demographics, preoperative investigation results, pathology findings, and operation details are shown in Table 1. Details of the histological findings are presented in Supplementary Table 1. The pancreatic duct size was obtained by measurement on CT (256 patients) or MRI (32 patients). The pancreatic duct size ranged from 1 mm to 12 mm with a median of 3 mm. The soft pancreatic texture was noted in 67.4% of patients. Majority of the patients (95.1%) received prophylactic Sandostatin perioperatively and an internal pancreatic stent was used in 13.5% of patients.
The operative outcomes are shown in Table 2. The overall pancreatic leak occurred in 56.3% of patients of which the majority were biochemical leaks (43.8%). Grade B and C pancreatic leak accounted for 8.7% and 1.4%, respectively. Overall operative morbidity was 51.4% of which 19.1% were major complications (Clavien-Dindo grade 3 or above). Seven (2.4%) patients underwent re-operation for PPH, anastomotic leak, or intestinal obstruction.
Five patients died within 90 days after operation. One patient died of myocardial infarction on postoperation day 8. Two patients developed PPH secondary to pancreatic leak and although the bleeding stopped after arterial embolization, one patient died of liver failure and the other died of bowel ischemia afterward. One patient had portal vein thrombosis postoperatively with re-operation; he died more than one month after surgery due to sepsis and bleeding. The last patient developed very early recurrent disease at two months postoperatively with carcinomatosis and intestinal obstruction.
A comparison was made between 29 patients with a clinically significant pancreatic leak (grade B and C) and 259 patients without leak (Table 3). Patients who developed POPF had significantly softer pancreas (p < 0.001), more carcinoma of the pancreas (p = 0.027), and smaller pancreatic duct size (p = 0.031). Univariate and multivariable analysis for clinically significant POPF was done by logistic regression using different pancreatic duct diameters. After excluding patients with hard pancreas who had a low risk of POPF, only a cut-off of pancreatic duct diameter ≤ 1 mm and > 1 mm was a significant predictive factor for POPF (p = 0.027; Table 4). It was a significant factor when the analysis included the hard pancreas (p = 0.028; Supplementary Table 2) or after excluding the 32 patients whose duct size were measured on CT or MRI (p = 0.036; Supplementary Table 3).
Fig. 2 shows the rate of POPF with different pancreatic duct sizes. Pancreatic duct size < 1 mm had the highest POPF rate while duct diameter > 4 to 5 mm had the lowest rate. When the POPF rate was compared between adjacent duct diameters, there was a significant difference between the ductal diameter ≤ 1 mm and > 1 mm to 2 mm (35.7% vs. 13.3%; p = 0.040). However, the difference was insignificant when the 32 patients with CT or MRI duct size measurement were excluded from the analysis (p = 0.068; Supplementary Fig. 1A) or when the 94 patients with hard pancreas were excluded from the analysis (p = 0.124; Supplementary Fig. 1B).
The pancreaticoenteric anastomosis is regarded as the Achilles heel as it is the most important part of PD operation and if unsuccessful the results can be detrimental especially due to POPF. The risk of POPF varies among different patients. A soft pancreas with a small pancreatic duct < 3 mm are considered high-risk factors for POPF [24]. The commonly used Fistula Risk Score (FRS) derived by Callery et al. [21] incorporates 4 baseline characteristics: pancreatic gland texture, pathology, pancreatic duct size, and intraoperative estimated blood loss of which the pancreatic duct size outscores the other three factors as the most important factor for POPF. It should be noted that in FRS there is a gradual increase in the risk of POPF with a gradual decrease in pancreatic duct size from ≥ 5 mm down to ≤ 1 mm.
Although there was an average of only 17 to 18 cases per year in our institute, the operative outcomes in this series are comparable with high volume centers, with a 90-day mortality of 1.7% and morbidity of 51.4% [25,26]. Clinical significant POPF occurred in 10.1% of the patients. Though there was no significant difference in the rate of POPF between the early and late periods of study, the POPF rate was higher in the late period of the study (Table 3). This was probably because more difficult cases were done in late period which could lead to increased pancreatic leak. PPH occurred in 5.6% of patients and seven patients (2.4%) required re-operation. Considering that the majority of our patients had a soft pancreas and the median pancreatic duct size was only 3mm, this study cohort represented a high-risk group of POPF.
Based on the results of the logistic regression for POPF with different pancreatic duct sizes ≤ 1 mm and > 1 mm was a significant factor for POPF, but pancreatic duct sizes of ≤ 2 mm or ≤ 3 mm were not significant for POPF. A more detailed analysis of POPF rate versus different pancreatic duct sizes (Fig. 2) showed that a pancreatic duct size of ≤ 1 mm had a significantly higher leak rate compared with others. Together all these results showed that for a pancreatic duct size of ≤ 1 mm, DTMPJ may not be a good choice of anastomosis. This is particularly because the DTM sutures were difficult to place in such an extremely small duct size. On the other hand, our results showed that for pancreatic duct size 2 or 3 mm, the DTMPJ efficiency and safety were comparable to that of larger duct size. In real life, for a duct size diameter of 2 mm, the circumference length would be 6.28 mm, this can accommodate 6 stitches at 1-mm apart. Therefore, anastomosis with surgical loupes, fine suturing instruments, and meticulous techniques, will have reduced challenges with enhanced efficiency and safety. However, a 1 mm or less pancreatic duct, can only accommodate 3 or 4 stitches and therefore it is technically extremely difficult to perform a DTM anastomosis, though 3 or 4 stitches are still required for the anastomosis.
Some authors have suggested the selection of different PJ techniques according to pancreatic texture and duct size, in brief, reserving DTM for duct > 5 mm [20]. In our experience, DTMPJ is still a reliable anastomosis in small ducts and the present study shows that it is safe for duct > 1 mm. However, for duct size < 1 mm, alternative techniques such as dunking or invagination PJ, binding PJ or PG should be considered since duct size does not add to the technical difficulties in these types of anastomosis [27-29]. Pancreatic duct occlusion with glue was also reported to be safe in the high-risk pancreatic stump with satisfactory early postoperative outcomes [30].
The main limitation of this study lies in its retrospective nature. Pancreatic duct size was not recorded by the operating surgeon in all the cases but was retrieved from the measurements on the preoperative CT or MRI; this might cause a discrepancy with the actual duct size during anastomosis. Furthermore, the pancreas might be transected at a site more towards the tail region rather than at the pancreatic neck where measurement of duct size was made on CT or MRI. Nevertheless, separate analysis excluding patients whose duct size were derived from measurement on CT or MRI yielded results that were similar to the whole group of patients (Table 3, Supplementary Table 3). The use of pancreatic stents and Sandostatin were not standardized in this study. Body mass index (BMI), which was a contributing factor for POPF and was included in an alternative risk score, was not recorded in our database [31]. Thus the impact of BMI on the incidence of POPF could not be evaluated. Finally, this study utilized one particular form of modified Blumgart’s technique for DTMPJ, the study result might not be applicable for other types of DTMPJ.
In summary, the present study showed that DTMPJ can be safely performed for pancreatic duct > 1 mm with a low risk of POPF. To the best of our knowledge, this is the first time a critical size of pancreatic duct was shown to affect the success of DTMPJ regarding the occurrence of POPF and this has an important bearing on how to handle the pancreatic stump after PD.
Supplementary data related to this article can be found at https://doi.org/10.14701/ahbps.21-054.
ACKNOWLEDGEMENTS
The authors thank Mr. Philip Ip for his assistance with data-keeping, processing, and statistical analysis
REFERENCES
1. Büchler MW, Wagner M, Schmied BM, Uhl W, Friess H, Z'graggen K. 2003; Changes in morbidity after pancreatic resection: toward the end of completion pancreatectomy. Arch Surg. 138:1310–1314. DOI: 10.1001/archsurg.138.12.1310. PMID: 14662530.
2. Kimura W, Miyata H, Gotoh M, Hirai I, Kenjo A, Kitagawa Y, et al. 2014; A pancreaticoduodenectomy risk model derived from 8575 cases from a national single-race population (Japanese) using a web-based data entry system: the 30-day and in-hospital mortality rates for pancreaticoduodenectomy. Ann Surg. 259:773–780. DOI: 10.1097/SLA.0000000000000263. PMID: 24253151.

3. Cameron JL, He J. 2015; Two thousand consecutive pancreaticoduodenectomies. J Am Coll Surg. 220:530–536. DOI: 10.1016/j.jamcollsurg.2014.12.031. PMID: 25724606.

4. Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, et al. 2017; The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery. 161:584–591. DOI: 10.1016/j.surg.2016.11.014. PMID: 28040257.

5. Pratt WB, Callery MP, Vollmer CM Jr. 2008; Risk prediction for development of pancreatic fistula using the ISGPF classification scheme. World J Surg. 32:419–428. DOI: 10.1007/s00268-007-9388-5. PMID: 18175170.

6. Addeo P, Delpero JR, Paye F, Oussoultzoglou E, Fuchshuber PR, Sauvanet A, et al. 2014; Pancreatic fistula after a pancreaticoduodenectomy for ductal adenocarcinoma and its association with morbidity: a multicentre study of the French Surgical Association. HPB (Oxford). 16:46–55. DOI: 10.1111/hpb.12063. PMID: 23461663. PMCID: PMC3892314.

7. Liu QY, Zhang WZ, Xia HT, Leng JJ, Wan T, Liang B, et al. 2014; Analysis of risk factors for postoperative pancreatic fistula following pancreaticoduodenectomy. World J Gastroenterol. 20:17491–17497. DOI: 10.3748/wjg.v20.i46.17491. PMID: 25516663. PMCID: PMC4265610.

8. Greene BS, Loubeau JM, Peoples JB, Elliott DW. 1991; Are pancreatoenteric anastomoses improved by duct-to-mucosa sutures? Am J Surg. 161:45–49. DOI: 10.1016/0002-9610(91)90359-L. PMID: 1987857.

9. Z'graggen K, Uhl W, Friess H, Büchler MW. 2002; How to do a safe pancreatic anastomosis. J Hepatobiliary Pancreat Surg. 9:733–737. DOI: 10.1007/s005340200101. PMID: 12658408.
10. Bassi C, Falconi M, Molinari E, Mantovani W, Butturini G, Gumbs AA, et al. 2003; Duct-to-mucosa versus end-to-side pancreaticojejunostomy reconstruction after pancreaticoduodenectomy: results of a prospective randomized trial. Surgery. 134:766–771. DOI: 10.1016/S0039-6060(03)00345-3. PMID: 14639354.

11. Berger AC, Howard TJ, Kennedy EP, Sauter PK, Bower-Cherry M, Dutkevitch S, et al. 2009; Does type of pancreaticojejunostomy after pancreaticoduodenectomy decrease rate of pancreatic fistula? A randomized, prospective, dual-institution trial. J Am Coll Surg. 208:738–747. DOI: 10.1016/j.jamcollsurg.2008.12.031. PMID: 19476827.

12. Mason GR, Freeark RJ. 1995; Current experience with pancreatogastrostomy. Am J Surg. 169:217–219. DOI: 10.1016/S0002-9610(99)80140-8. PMID: 7840383.

13. Gómez T, Palomares A, Serradilla M, Tejedor L. 2014; Reconstruction after pancreatoduodenectomy: pancreatojejunostomy vs pancreatogastrostomy. World J Gastrointest Oncol. 6:369–376. DOI: 10.4251/wjgo.v6.i9.369. PMID: 25232462. PMCID: PMC4163735.

14. Wang W, Zhang Z, Gu C, Liu Q, Liang Z, He W, et al. 2018; The optimal choice for pancreatic anastomosis after pancreaticoduodenectomy: a network meta-analysis of randomized control trials. Int J Surg. 57:111–116. DOI: 10.1016/j.ijsu.2018.04.005. PMID: 29777880.

15. Jin Y, Feng YY, Qi XG, Hao G, Yu YQ, Li JT, et al. 2019; Pancreatogastrostomy vs pancreatojejunostomy after pancreaticoduodenectomy: an updated meta-analysis of RCTs and our experience. World J Gastrointest Surg. 11:322–332. DOI: 10.4240/wjgs.v11.i7.322. PMID: 31602291. PMCID: PMC6783689.

16. Shrikhande SV, Sivasanker M, Vollmer CM, Friess H, Besselink MG, Fingerhut A, et al. 2017; Pancreatic anastomosis after pancreatoduodenectomy: a position statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 161:1221–1234. DOI: 10.1016/j.surg.2016.11.021. PMID: 28027816.

17. Kleespies A, Rentsch M, Seeliger H, Albertsmeier M, Jauch KW, Bruns CJ. 2009; Blumgart anastomosis for pancreaticojejunostomy minimizes severe complications after pancreatic head resection. Br J Surg. 96:741–750. DOI: 10.1002/bjs.6634. PMID: 19526614.

18. Grobmyer SR, Kooby D, Blumgart LH, Hochwald SN. 2010; Novel pancreaticojejunostomy with a low rate of anastomotic failure-related complications. J Am Coll Surg. 210:54–59. DOI: 10.1016/j.jamcollsurg.2009.09.020. PMID: 20123332.

19. Hirono S, Kawai M, Okada KI, Miyazawa M, Kitahata Y, Hayami S, et al. 2019; Modified Blumgart mattress suture versus conventional interrupted suture in pancreaticojejunostomy during pancreaticoduodenectomy: randomized controlled trial. Ann Surg. 269:243–251. DOI: 10.1097/SLA.0000000000002802. PMID: 29697455. PMCID: PMC6325750.

20. Suzuki Y, Fujino Y, Tanioka Y, Hiraoka K, Takada M, Ajiki T, et al. 2002; Selection of pancreaticojejunostomy techniques according to pancreatic texture and duct size. Arch Surg. 137:1044–1047. DOI: 10.1001/archsurg.137.9.1044. PMID: 12215157.

21. Callery MP, Pratt WB, Kent TS, Chaikof EL, Vollmer CM Jr. 2013; A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J Am Coll Surg. 216:1–14. DOI: 10.1016/j.jamcollsurg.2012.09.002. PMID: 23122535.

22. Dindo D, Demartines N, Clavien PA. 2004; Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 240:205–213. DOI: 10.1097/01.sla.0000133083.54934.ae. PMID: 15273542. PMCID: PMC1360123.
23. Swanson RS, Pezzi CM, Mallin K, Loomis AM, Winchester DP. 2014; The 90-day mortality after pancreatectomy for cancer is double the 30-day mortality: more than 20,000 resections from the national cancer data base. Ann Surg Oncol. 21:4059–4067. DOI: 10.1245/s10434-014-4036-4. PMID: 25190121.

24. Shukla PJ, Barreto SG, Fingerhut A, Bassi C, Büchler MW, Dervenis C, et al. 2010; Toward improving uniformity and standardization in the reporting of pancreatic anastomoses: a new classification system by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 147:144–153. DOI: 10.1016/j.surg.2009.09.003. PMID: 19879614.

25. Gouma DJ, van Geenen RC, van Gulik TM, de Haan RJ, de Wit LT, Busch OR, et al. 2000; Rates of complications and death after pancreaticoduodenectomy: risk factors and the impact of hospital volume. Ann Surg. 232:786–795. DOI: 10.1097/00000658-200012000-00007. PMID: 11088073. PMCID: PMC1421271.

26. Gooiker GA, van Gijn W, Wouters MW, Post PN, van de Velde CJ, Tollenaar RA. 2011; Systematic review and meta-analysis of the volume-outcome relationship in pancreatic surgery. Br J Surg. 98:485–494. DOI: 10.1002/bjs.7413. PMID: 21500187.

27. Cheng Y, Briarava M, Lai M, Wang X, Tu B, Cheng N, et al. 2017; Pancreaticojejunostomy versus pancreaticogastrostomy reconstruction for the prevention of postoperative pancreatic fistula following pancreaticoduodenectomy. Cochrane Database Syst Rev. 9:CD012257. DOI: 10.1002/14651858.CD012257.pub2. PMID: 28898386. PMCID: PMC6483797.

28. Lyu Y, Li T, Wang B, Cheng Y, Zhao S. 2018; Selection of pancreaticojejunostomy technique after pancreaticoduodenectomy: duct-to-mucosa anastomosis is not better than invagination anastomosis: a meta-analysis. Medicine (Baltimore). 97:e12621. DOI: 10.1097/MD.0000000000012621. PMID: 30290634. PMCID: PMC6200508.
29. Olakowski M, Grudzińska E, Mrowiec S. 2020; Pancreaticojejunostomy-a review of modern techniques. Langenbecks Arch Surg. 405:13–22. DOI: 10.1007/s00423-020-01855-6. PMID: 31975148. PMCID: PMC7036071.

30. Mazzaferro V, Virdis M, Sposito C, Cotsoglou C, Droz Dit Busset M, Bongini M, et al. 2019; Permanent pancreatic duct occlusion with neoprene-based glue injection after pancreatoduodenectomy at high risk of pancreatic fistula: a prospective clinical study. Ann Surg. 270:791–798. DOI: 10.1097/SLA.0000000000003514. PMID: 31567180. PMCID: PMC6867669.

31. Mungroop TH, van Rijssen LB, van Klaveren D, Smits FJ, van Woerden V, Linnemann RJ, et al. 2019; Alternative fistula risk score for pancreatoduodenectomy (a-FRS): design and international external validation. Ann Surg. 269:937–943. DOI: 10.1097/SLA.0000000000002620. PMID: 29240007.
Fig. 1
A Schematic drawing of the modified Blumgart’s technique. (A) Posterior layer of the outer transpancreatic U-sutures were placed. (B) Posterior layer of duct-to-mucosa interrupted stitches were tied after the U-sutures were tightened, anterior row of duct-to-mucosa stitches were inserted. (C) After the anterior row of duct-to-mucosa were tightened and tied, the anterior row of the outer U-suture were made. (D) Completed anastomosis after the U-sutures were tied.
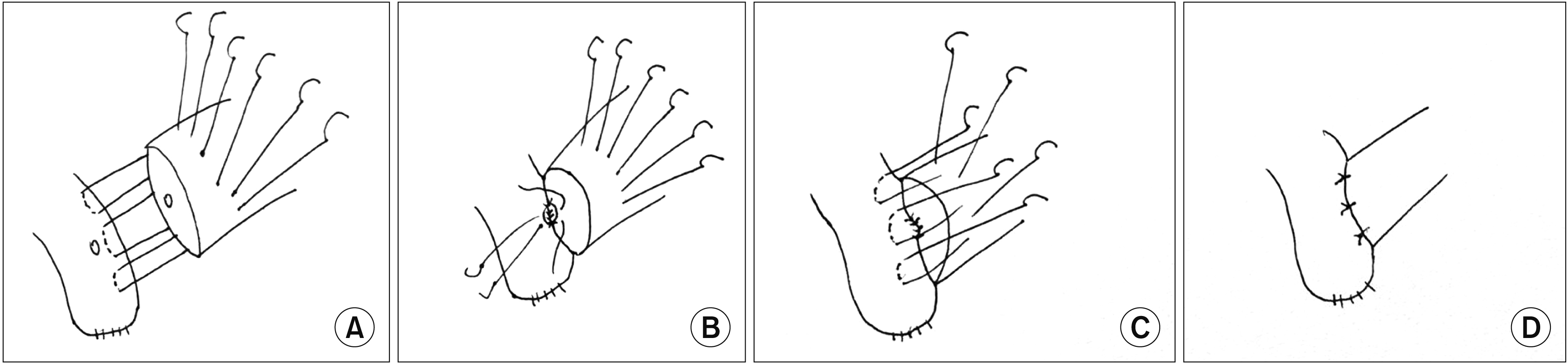
Fig. 2
The relationship between pancreatic duct size and clinical significant pancreatic fistula. *Statistically significant (p < 0.05).
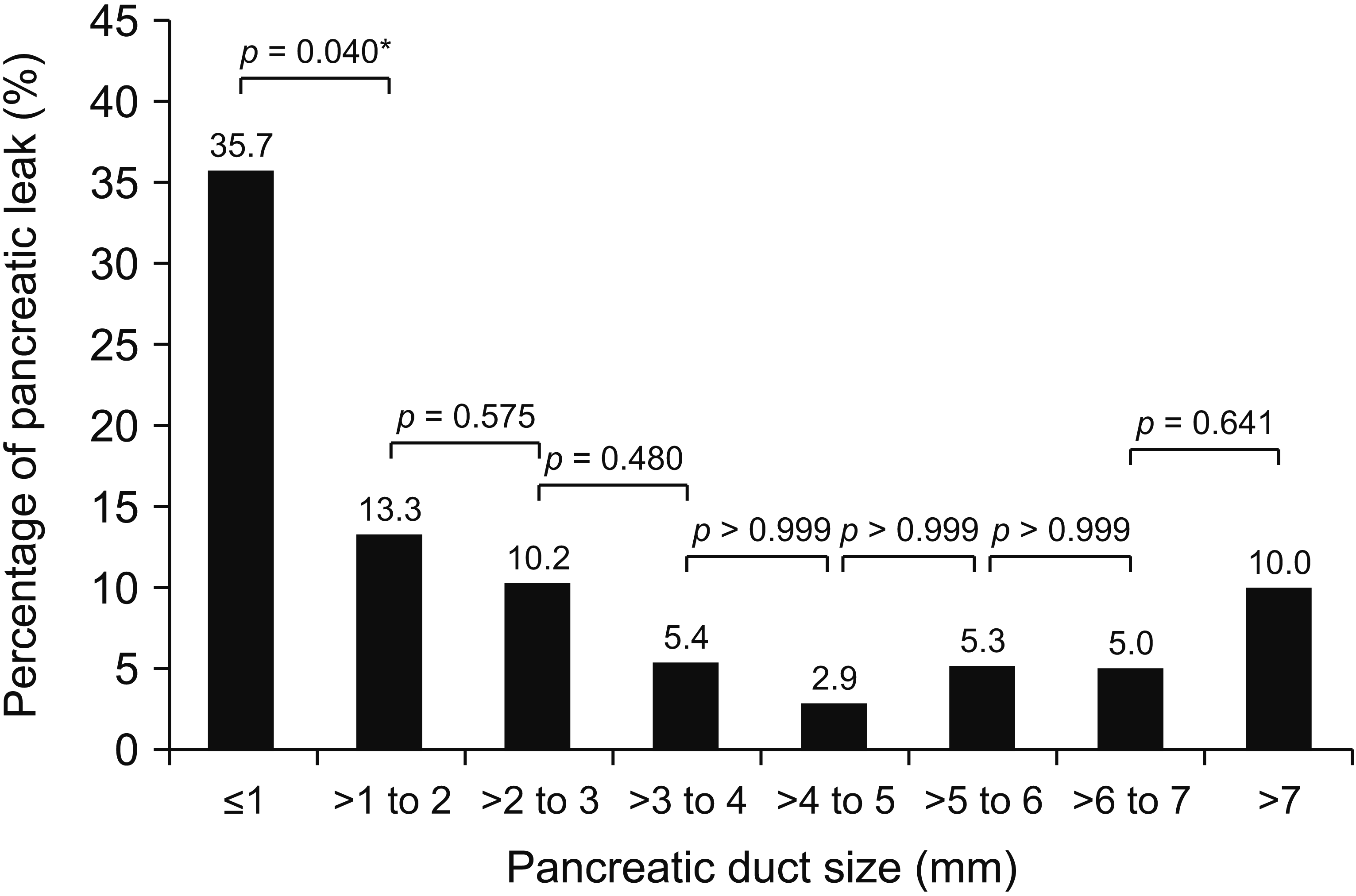
Table 1
Patients’ demographics, preoperative investigation results, pathology findings, and operative characteristics (n = 288)
Table 2
Operative outcomes (n = 288)
Table 3
A comparison between patients with and without postoperative pancreatic fistula
| Variable | Clinical significant pancreatic fistula | p-value | |
|---|---|---|---|
|
|
|||
| No (n = 259) | Yes (n = 29) | ||
| Study period | 0.195 | ||
| Jan 2003 to Jun 2011 | 74 (93.7) | 5 (6.3) | |
| Jul 2011 to Dec 2019 | 185 (88.5) | 24 (11.5) | |
| Age (yr) | 63.3 ± 10.4 | 65.8 ± 8.9 | 0.213 |
| Sex | 0.611 | ||
| Male | 148 (57.1) | 18 (62.1) | |
| Female | 111 (42.9) | 11 (37.9) | |
| ASA | 0.489 | ||
| 1 | 41 (15.8) | 5 (17.2) | |
| 2 | 167 (64.5) | 16 (55.2) | |
| 3 | 51 (19.7) | 8 (27.6) | |
| Hemoglobin (g/dL) | 12.2 ± 1.6 | 12.7 ± 1.9 | 0.094 |
| Platelet (× 109/L) | 261.6 ± 89.8 | 264.8 ± 110.5 | 0.860 |
| White cell count (× 109/L) | 6.8 ± 2.4 | 7.8 ± 4.0 | 0.059 |
| International normalized ratio | 1.01 ± 0.08 | 0.98 ± 0.07 | 0.116 |
| Creatinine (mmol/L) | 75.0 ± 21.7 | 83.3 ± 25.0 | 0.054 |
| Albumin (g/L) | 36.5 ± 5.8 | 35.1 ± 6.8 | 0.237 |
| Bilirubin (mmol/L) | 58.8 ± 91.2 | 67.9 ± 114.0 | 0.621 |
| Alkaline phosphatase (IU/L) | 213.1 ± 207.3 | 161.4 ± 135.2 | 0.199 |
| Pancreas texture | < 0.001* | ||
| Soft | 166 (64.1) | 28 (96.6) | |
| Hard | 93 (35.9) | 1 (3.4) | |
| Carcinoma of pancreas | 88 (34.0) | 4 (13.8) | 0.027* |
| Pancreatic duct size (mm) | 4.1 ± 2.2 | 3.2 ± 2.2 | 0.031* |
| Pancreatic duct size (mm) (exclude duct measurement by CT or MRI) | 4.2 ±2.3 (n = 230) | 3.3 ± 2.3 (n = 26) | 0.046* |
| Operative approach | 0.577 | ||
| Open | 252 (97.3) | 28 (96.6) | |
| Laparoscopic/robotic to open | 3 (1.2) | 1 (3.4) | |
| Robotic | 4 (1.5) | 0 (0.0) | |
| Concomitant procedure | 10 (3.9) | 2 (6.9) | 0.345 |
| Use of pancreatic stent | 35 (13.5) | 4 (13.8) | > 0.999 |
| Prophylactic Sandostatin | 246 (95.0) | 28 (96.6) | > 0.999 |
| Operative time (min) | 530.1 ± 84.1 | 541.9 ± 74.5 | 0.472 |
| Blood loss (mL) | 697.2 ± 822.1 | 1,125.3 ± 1,986.5 | 0.260 |
| Vascular resection | 13 (5.0) | 1 (3.4) | > 0.999 |
| Blood transfusion | 52 (20.1) | 9 (31.0) | 0.171 |
Table 4
Logistic regression for clinical significant pancreatic fistula with different pancreatic duct sizes cut off (excluding patients with hard pancreas)
| Variable | Univariate | Multivariable | |||
|---|---|---|---|---|---|
|
|
|
||||
| Odds ratio (95% CI) | p-value | Odds ratio (95% CI) | p-value | ||
| Pancreatic duct as continuous variable | |||||
| Carcinoma of pancreas | 0.710 (0.199–2.537) | 0.598 | - | ||
| Pancreatic duct size (mm) | 0.940 (0.755–1.171) | 0.581 | - | ||
| Pancreatic duct (> 1 mm vs. ≤ 1 mm) | |||||
| Carcinoma of pancreas | 0.710 (0.199–2.537) | 0.598 | - | ||
| Pancreatic duct size (mm) | |||||
| ≤ 1 | 3.792 (1.168–12.311) | 0.027* | 3.792 (1.168–12.311) | 0.027* | |
| > 1 | - | - | |||




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download