1. Lafaro KJ, Demirjian AN, Pawlik TM. 2015; Epidemiology of hepatocellular carcinoma. Surg Oncol Clin N Am. 24:1–17. DOI:
10.1016/j.soc.2014.09.001. PMID:
25444466.

2. Fujiwara N, Friedman SL, Goossens N, Hoshida Y. 2018; Risk factors and prevention of hepatocellular carcinoma in the era of precision medicine. J Hepatol. 68:526–549. DOI:
10.1016/j.jhep.2017.09.016. PMID:
28989095. PMCID:
PMC5818315.

3. Petrick JL, Kelly SP, Altekruse SF, McGlynn KA, Rosenberg PS. 2016; Future of hepatocellular carcinoma incidence in the United States forecast through 2030. J Clin Oncol. 34:1787–1794. DOI:
10.1200/JCO.2015.64.7412. PMID:
27044939. PMCID:
PMC4966339.

4. McGlynn KA, Petrick JL, London WT. 2015; Global epidemiology of hepatocellular carcinoma: an emphasis on demographic and regional variability. Clin Liver Dis. 19:223–238. DOI:
10.1016/j.cld.2015.01.001. PMID:
25921660. PMCID:
PMC4712629.
5. El-Serag HB, Siegel AB, Davila JA, Shaib YH, Cayton-Woody M, McBride R, et al. 2006; Treatment and outcomes of treating of hepatocellular carcinoma among Medicare recipients in the United States: a population-based study. J Hepatol. 44:158–166. DOI:
10.1016/j.jhep.2005.10.002. PMID:
16290309.

6. op den Winkel M, Nagel D, Sappl J, op den Winkel P, Lamerz R, Zech CJ, et al. 2012; Prognosis of patients with hepatocellular carcinoma. Validation and ranking of established staging-systems in a large western HCC-cohort. PLoS One. 7:e45066. DOI:
10.1371/journal.pone.0045066. PMID:
23071507. PMCID:
PMC3465308.

8. Halazun KJ, Najjar M, Abdelmessih RM, Samstein B, Griesemer AD, Guarrera JV, et al. 2017; Recurrence after liver transplantation for hepatocellular carcinoma: a new MORAL to the story. Ann Surg. 265:557–564. DOI:
10.1097/SLA.0000000000001966. PMID:
27611615.
9. Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, et al. 2001; Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 33:1394–1403. DOI:
10.1053/jhep.2001.24563. PMID:
11391528.

10. Krenzien F, Schmelzle M, Struecker B, Raschzok N, Benzing C, Jara M, et al. 2018; Liver transplantation and liver resection for cirrhotic patients with hepatocellular carcinoma: comparison of long-term survivals. J Gastrointest Surg. 22:840–848. DOI:
10.1007/s11605-018-3690-4. PMID:
29363019.

11. Zheng Z, Liang W, Milgrom DP, Zheng Z, Schroder PM, Kong NS, et al. 2014; Liver transplantation versus liver resection in the treatment of hepatocellular carcinoma: a meta-analysis of observational studies. Transplantation. 97:227–234. DOI:
10.1097/TP.0b013e3182a89383. PMID:
24142034.

12. Pinna AD, Yang T, Mazzaferro V, De Carlis L, Zhou J, Roayaie S, et al. 2018; Liver transplantation and hepatic resection can achieve cure for hepatocellular carcinoma. Ann Surg. 268:868–875. DOI:
10.1097/SLA.0000000000002889. PMID:
30080736.

13. Tsilimigras DI, Bagante F, Moris D, Merath K, Paredes AZ, Sahara K, et al. 2019; Defining the chance of cure after resection for hepatocellular carcinoma within and beyond the Barcelona Clinic Liver Cancer guidelines: a multi-institutional analysis of 1,010 patients. Surgery. 166:967–974. DOI:
10.1016/j.surg.2019.08.010. PMID:
31606196.

14. Lambert PC, Thompson JR, Weston CL, Dickman PW. 2007; Estimating and modeling the cure fraction in population-based cancer survival analysis. Biostatistics. 8:576–594. DOI:
10.1093/biostatistics/kxl030. PMID:
17021277.

16. Guyot P, Ades AE, Ouwens MJ, Welton NJ. 2012; Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 12:9. DOI:
10.1186/1471-2288-12-9. PMID:
22297116. PMCID:
PMC3313891.

17. R Development Core Team. R: a language and environment for statistical computing [Internet]. Available from:
https://www.R-project.org/. Vienna: R Foundation for Statistical Computing;2017. cited 2020 Dec 1.
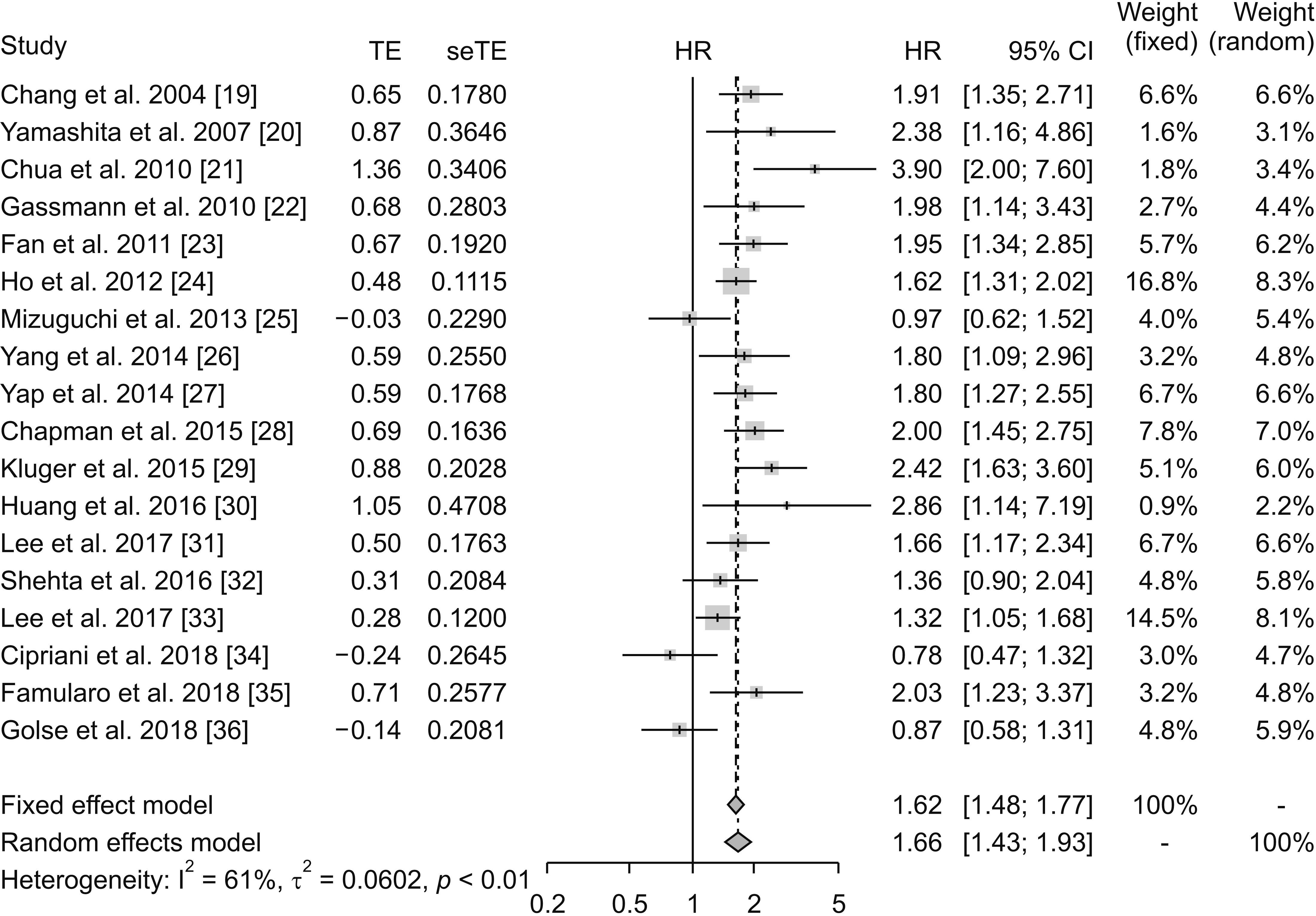
19. Chang CH, Chau GY, Lui WY, Tsay SH, King KL, Wu CW. 2004; Long-term results of hepatic resection for hepatocellular carcinoma originating from the noncirrhotic liver. Arch Surg. 139:320–325. DOI:
10.1001/archsurg.139.3.320. PMID:
15006892.

20. Yamashita Y, Taketomi A, Itoh S, Kitagawa D, Kayashima H, Harimoto N, et al. 2007; Longterm favorable results of limited hepatic resections for patients with hepatocellular carcinoma: 20 years of experience. J Am Coll Surg. 205:19–26. DOI:
10.1016/j.jamcollsurg.2007.01.069. PMID:
17617328.

21. Chua TC, Saxena A, Chu F, Liauw W, Zhao J, Morris DL. 2010; Clinicopathological determinants of survival after hepatic resection of hepatocellular carcinoma in 97 patients--experience from an Australian hepatobiliary unit. J Gastrointest Surg. 14:1370–1380. DOI:
10.1007/s11605-010-1277-9. PMID:
20585991.

22. Gassmann P, Spieker T, Haier J, Schmidt F, Mardin WA, Senninger N. 2010; Prognostic impact of underlying liver fibrosis and cirrhosis after curative resection of hepatocellular carcinoma. World J Surg. 34:2442–2451. DOI:
10.1007/s00268-010-0655-5. PMID:
20544346.

23. Fan ST, Poon RT, Yeung C, Lam CM, Lo CM, Yuen WK, et al. 2011; Outcome after partial hepatectomy for hepatocellular cancer within the Milan criteria. Br J Surg. 98:1292–1300. DOI:
10.1002/bjs.7583. PMID:
21656513.

24. Ho CM, Lee PH, Chen CL, Ho MC, Wu YM, Hu RH. 2012; Long-term outcomes after resection versus transplantation for hepatocellular carcinoma within UCSF criteria. Ann Surg Oncol. 19:826–833. DOI:
10.1245/s10434-011-1975-x. PMID:
21879276.

25. Mizuguchi T, Kawamoto M, Meguro M, Nakamura Y, Ota S, Hui TT, et al. 2013; Prognosis and predictors of surgical complications in hepatocellular carcinoma patients with or without cirrhosis after hepatectomy. World J Surg. 37:1379–1387. DOI:
10.1007/s00268-013-1989-6. PMID:
23479099.

26. Yang LY, Chang RM, Lau WY, Ou DP, Wu W, Zeng ZJ. 2014; Mesohepatectomy for centrally located large hepatocellular carcinoma: indications, techniques, and outcomes. Surgery. 156:1177–1187. DOI:
10.1016/j.surg.2014.05.012. PMID:
25444316.

27. Yap AQ, Millan CA, Wang JH, Wang CC, Lu SN, Wang SH, et al. 2014; How to improve the outcome in patients with AJCC stage I hepatocellular carcinoma. Anticancer Res. 34:3093–3103. PMID:
24922678.
28. Chapman WC, Klintmalm G, Hemming A, Vachharajani N, Majella Doyle MB, DeMatteo R, et al. 2015; Surgical treatment of hepatocellular carcinoma in North America: can hepatic resection still be justified? J Am Coll Surg. 220:628–637. DOI:
10.1016/j.jamcollsurg.2014.12.030. PMID:
25728142.

29. Kluger MD, Salceda JA, Laurent A, Tayar C, Duvoux C, Decaens T, et al. 2015; Liver resection for hepatocellular carcinoma in 313 Western patients: tumor biology and underlying liver rather than tumor size drive prognosis. J Hepatol. 62:1131–1140. DOI:
10.1016/j.jhep.2014.12.018. PMID:
25529622.

30. Huang ZY, Liang BY, Xiong M, Dong KS, Zhang ZY, Zhang EL, et al. 2016; Severity of cirrhosis should determine the operative modality for patients with early hepatocellular carcinoma and compensated liver function. Surgery. 159:621–631. DOI:
10.1016/j.surg.2015.09.002. PMID:
26453137.

31. Lee HW, Choi GH, Kim DY, Park YN, Kim KS, Choi JS, et al. 2017; Less fibrotic burden differently affects the long-term outcomes of hepatocellular carcinoma after curative resection. Oncology. 93:224–232. DOI:
10.1159/000477173. PMID:
28571030.

32. Shehta A, Han HS, Yoon YS, Cho JY, Choi Y. 2016; Laparoscopic liver resection for hepatocellular carcinoma in cirrhotic patients: 10-year single-center experience. Surg Endosc. 30:638–648. DOI:
10.1007/s00464-015-4253-3. PMID:
26091992.

33. Lee EC, Kim SH, Park H, Lee SD, Lee SA, Park SJ. 2017; Survival analysis after liver resection for hepatocellular carcinoma: a consecutive cohort of 1002 patients. J Gastroenterol Hepatol. 32:1055–1063. DOI:
10.1111/jgh.13632. PMID:
27797420.

34. Cipriani F, Fantini C, Ratti F, Lauro R, Tranchart H, Halls M, et al. 2018; Laparoscopic liver resections for hepatocellular carcinoma. Can we extend the surgical indication in cirrhotic patients? Surg Endosc. 32:617–626. DOI:
10.1007/s00464-017-5711-x. PMID:
28717870.

35. Famularo S, Di Sandro S, Giani A, Lauterio A, Sandini M, De Carlis R, et al. 2018; Long-term oncologic results of anatomic vs. parenchyma-sparing resection for hepatocellular carcinoma. A propensity score-matching analysis. Eur J Surg Oncol. 44:1580–1587. DOI:
10.1016/j.ejso.2018.05.018. PMID:
29861336.

36. Golse N, El Bouyousfi A, Marques F, Bancel B, Mohkam K, Ducerf C, et al. 2018; Large hepatocellular carcinoma: does fibrosis really impact prognosis after resection? J Visc Surg. 155:265–273. DOI:
10.1016/j.jviscsurg.2017.10.015. PMID:
29525540.

37. Akcam AT, Saritas AG, Ulku A, Rencuzogullari A. 2019; Oncological outcomes of hepatic resection vs transplantation for localized hepatocellular carcinoma. Transplant Proc. 51:1147–1152. DOI:
10.1016/j.transproceed.2019.01.093. PMID:
31101189.

38. Xu XF, Xing H, Han J, Li ZL, Lau WY, Zhou YH, et al. 2019; Risk factors, patterns, and outcomes of late recurrence after liver resection for hepatocellular carcinoma: a multicenter study from China. JAMA Surg. 154:209–217. DOI:
10.1001/jamasurg.2018.4334. PMID:
30422241. PMCID:
PMC6439634.

39. Li ZL, Yu JJ, Guo JW, Sui CJ, Dai BH, Zhang WG, et al. 2019; Liver resection is justified for multinodular hepatocellular carcinoma in selected patients with cirrhosis: a multicenter analysis of 1,066 patients. Eur J Surg Oncol. 45:800–807. DOI:
10.1016/j.ejso.2018.12.016. PMID:
30594407.

41. Fattovich G, Stroffolini T, Zagni I, Donato F. 2004; Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 127(5 Suppl 1):S35–S50. DOI:
10.1053/j.gastro.2004.09.014. PMID:
15508101.

42. Ju MJ, Qiu SJ, Fan J, Xiao YS, Gao Q, Zhou J, et al. 2009; Peritumoral activated hepatic stellate cells predict poor clinical outcome in hepatocellular carcinoma after curative resection. Am J Clin Pathol. 131:498–510. DOI:
10.1309/AJCP86PPBNGOHNNL. PMID:
19289585.

43. Yu DC, Liu J, Chen J, Shao JJ, Shen X, Xia HG, et al. 2014; GGPPS1 predicts the biological character of hepatocellular carcinoma in patients with cirrhosis. BMC Cancer. 14:248. DOI:
10.1186/1471-2407-14-248. PMID:
24716791. PMCID:
PMC4028285.

44. Lin Y, Liang R, Ye J, Li Q, Liu Z, Gao X, et al. 2019; A twenty gene-based gene set variation score reflects the pathological progression from cirrhosis to hepatocellular carcinoma. Aging (Albany NY). 11:11157–11169. DOI:
10.18632/aging.102518. PMID:
31811111. PMCID:
PMC6932912.

45. Harimoto N, Yoshizumi T, Fujimoto Y, Motomura T, Mano Y, Toshima T, et al. 2018; Surgery for hepatocellular carcinoma in patients with Child-Pugh B cirrhosis: hepatic resection versus living donor liver transplantation. World J Surg. 42:2606–2616. DOI:
10.1007/s00268-018-4493-1. PMID:
29372372.

46. Margarit C, Escartín A, Castells L, Vargas V, Allende E, Bilbao I. 2005; Resection for hepatocellular carcinoma is a good option in Child-Turcotte-Pugh class A patients with cirrhosis who are eligible for liver transplantation. Liver Transpl. 11:1242–1251. DOI:
10.1002/lt.20398. PMID:
16184539.

48. Kulik L, Heimbach JK, Zaiem F, Almasri J, Prokop LJ, Wang Z, et al. 2018; Therapies for patients with hepatocellular carcinoma awaiting liver transplantation: a systematic review and meta-analysis. Hepatology. 67:381–400. DOI:
10.1002/hep.29485. PMID:
28859222.

49. Chedid MF, Kruel CRP, Pinto MA, Grezzana-Filho TJM, Leipnitz I, Kruel CDP, et al. 2017; Hepatocellular carcinoma: diagnosis and operative management. Arq Bras Cir Dig. 30:272–278. DOI:
10.1590/0102-6720201700040011. PMID:
29340553. PMCID:
PMC5793147.

51. Chan DL, Alzahrani NA, Morris DL, Chua TC. 2014; Systematic review of efficacy and outcomes of salvage liver transplantation after primary hepatic resection for hepatocellular carcinoma. J Gastroenterol Hepatol. 29:31–41. DOI:
10.1111/jgh.12399. PMID:
24117517.

52. Muaddi H, Al-Adra DP, Beecroft R, Ghanekar A, Moulton CA, Doyle A, et al. 2018; Liver transplantation is equally effective as a salvage therapy for patients with hepatocellular carcinoma recurrence following radiofrequency ablation or liver resection with curative intent. Ann Surg Oncol. 25:991–999. DOI:
10.1245/s10434-017-6329-x. PMID:
29327179.

53. Qu W, Zhu ZJ, Sun LY, Wei L, Liu Y, Zeng ZG. 2015; Salvage liver transplantation for hepatocellular carcinoma recurrence after primary liver resection. Clin Res Hepatol Gastroenterol. 39:93–97. DOI:
10.1016/j.clinre.2014.07.006. PMID:
25150375.

54. Zheng S, Xie Q, Cheng J. 2018; Salvage liver transplant for hepatocellular carcinoma: rescues and benefits. Transl Gastroenterol Hepatol. 3:65. DOI:
10.21037/tgh.2018.09.06. PMID:
30363838. PMCID:
PMC6182042.

56. Chan AC, Chan SC, Chok KS, Cheung TT, Chiu DW, Poon RT, et al. 2013; Treatment strategy for recurrent hepatocellular carcinoma: salvage transplantation, repeated resection, or radiofrequency ablation? Liver Transpl. 19:411–419. DOI:
10.1002/lt.23605. PMID:
23447460.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download