INTRODUCTION
MATERIALS AND METHODS
Search terms
Inclusion criteria
Definitions
Outcomes evaluated
Data extraction and quality assessment
Types of studies
Statistical analysis
RESULTS
Included studies
Table 1
| Author (year) | Study period | Total (n) | Follow-up time (mon) | R-status | % debulking | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| Curative (R0-R1) | Debulking (R2) | ≥ 90% | 90%–99% | ≥ 70% | 70%–79% | < 70% | |||||
| Graff-Baker et al. (2014) [22] | 2007–2011 | 52 | 38 | 19 (37%) | 33 (63%) | - | 22 (42%) | - | 11 (21%) | - | |
| Maxwell et al. (2016) [13] | 1999–2015 |
P: 28 SB: 80 |
49 | NR | NR |
P: 10 (28%) SB: 32 (32%) |
- |
P: 18 (50%) SB: 51 (51%) |
- |
P: 8 (22%) SB: 17 (17%) |
|
| Woltering et al. (2017) [15] | 2003–2016 |
P: 83c) SB: 487c) |
Mean: 79 | 99%–100%: P: 36 (43%) SB: 207 (43%) | < 99%: P: 47 (57%) SB: 280 (57%) |
P: 15 (18%)d) SB: 113 (23%)d) |
P: 32 (39%)d) SB: 48 (10%)d) |
P: NRd) SB: 119 (24%)d) |
|||
| Morgan et al. (2018) [14] | 2006–2016 | 44/42a) | 33 | 24 (55%) | 20 (45%) | - | 12 (27%) | - | 8 (18%) | - | |
| Chamberlain et al. (2000) [23] | 1992–1998 | 34b) | 27 | 15 (44%) | 19 (56%) | - | - | - | - | - | |
| Ejaz et al. (2018) [24] | 1990–2014 | 612 | 51 | 433 (71%) | 179 (29%) | - | - | - | - | - | |
| Elias et al. (2003) [12] | 1985–2000 | 47 | 62 | 37 (79%) | 10 (21%) | - | - | - | - | - | |
| Glazer et al. (2010) [10] | 1978–2009 | 140b) | 50 | R0: 117 (84%) | R1/2: 22 (16%) | - | - | - | - | - | |
| Scott et al. (2019) [16] | 1999–2017 | 188/184a) | 29 | NR | NR | 54 (31%)e) | - | - | 82 (48%)e) | 36 (21%)e) | |
| Nave et al. (2001) [25] | 1983–1996 | 31 | 42 | 10 (32%) | 21 (68%) | - | - | - | - | - | |
| Osborne et al. (2006) [26] | 2000–2004 | 61b) | NR | 38 (62%) | 23 (38%) | - | - | - | - | - | |
| Que et al. (1995) [20] | 1984–1992 | 74 | 26 | 28 (38%) | 46 (62%) | - | - | - | - | - | |
| Wängberg et al. (1996) [21] | NR | 64 | NR | 14 (22%) | 50 (78%) | - | - | - | - | - | |
a)Some patients underwent multiple resections, hence numbers are reported as “no. of resections/patients”. b)The number of patients treated with resection. c)The number of patients for which the volume of tumor resected was recorded. d)Woltering et al. [15] grouped patients as 90%–98% and < 90% for P, and as 90%–98%, 70%–89% and < 70% for SB. e) Scott et al. [16] grouped the patients as > 90%, 70%–90%, and < 70% debulking.
Curative vs. debulking surgery
Table 2
| Author | Overall | Five-year OS (median) | Univariable analysis of R2 vs. R0-R1 | ||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| Extent of debulking (R2) | Curative (R0-R1) | Debulking (R2) | HR (95% CI) | p-value | |||
| Chamberlain et al. [23] | N/A | 85% | 63% | - | 0.18 | ||
| Ejaz et al. [24] | ≥ 80% (liver-specific) | 85.2% (not reached) | 60.7% (7.3 yr) | 3.63 (2.35–5.62)a) | < 0.001* | ||
| Elias et al. [12] | ≥ 97% |
R0: 74% R1: 70% |
47% |
vs. R0: 2.6 (0.2–28.7)a) vs. R1: 2.5 (0.5–12.5)a) |
0.44b) | ||
| Glazer et al. [10] | ≥ 90% |
R0: N/A R1: N/A |
N/A | - | 0.4 | ||
| Morgan et al. [14] |
≥ 90% ≥ 70% |
R0: N/A |
90-99%: N/A 70-99%: N/A |
- | 0.64 0.45 | ||
| Nave et al. [25] | N/A (liver-specific) | R0: 86% | 26% | vs. R0: 3.90 (1.76–8.64)a) | 0.001* | ||
| Osborne et al. [26] | ≥ 90% | (Mean: 4.2 yr) | (Mean: 2.7 yr) | 3.10 (0.91–10.52)a) | < 0.01* | ||
| Que et al. [20] | N/A | N/A | N/A | 2.73 (0.84–8.93)a) | NS | ||
| Wängberg et al. [21] | N/A | R0: 100% | 63% | vs. R0: 3.74 (1.28–10.96)a) | N/A | ||
| Woltering et al. [15] (small bowel) |
90%–98% 70%–89% |
95% |
90%–98%: 87% 70%–89%: 89% |
vs. 90%–98%: 2.26 (1.29–3.96)a) vs. 70%–89%: 3.27 (2.02–5.29)a) |
N/A | ||
| Woltering et al. [15] (pancreas) |
90%–98% < 90% |
84% |
90%–98%: 68% < 90%: 56% |
vs. 90%–98%: 3.00 (0.57–15.76)a) vs. < 90%: 4.06 (1.11–14.93)a) |
N/A | ||
| Graff-Baker et al. [22]c) | 90%–99%, 70%–89% | N/Ac) | N/Ac) | - | 0.93c) | ||
The extent of debulking is based on the overall R-status, except for the stated studies, which used the liver-specific R-status. Survival estimates are reported as rates at five years and/or medians, and are for the combined R0-R1 group and the R2 group, unless stated otherwise. HRs are for debulking (R2) vs. curative (R0-R1), unless stated otherwise.
Fig. 2
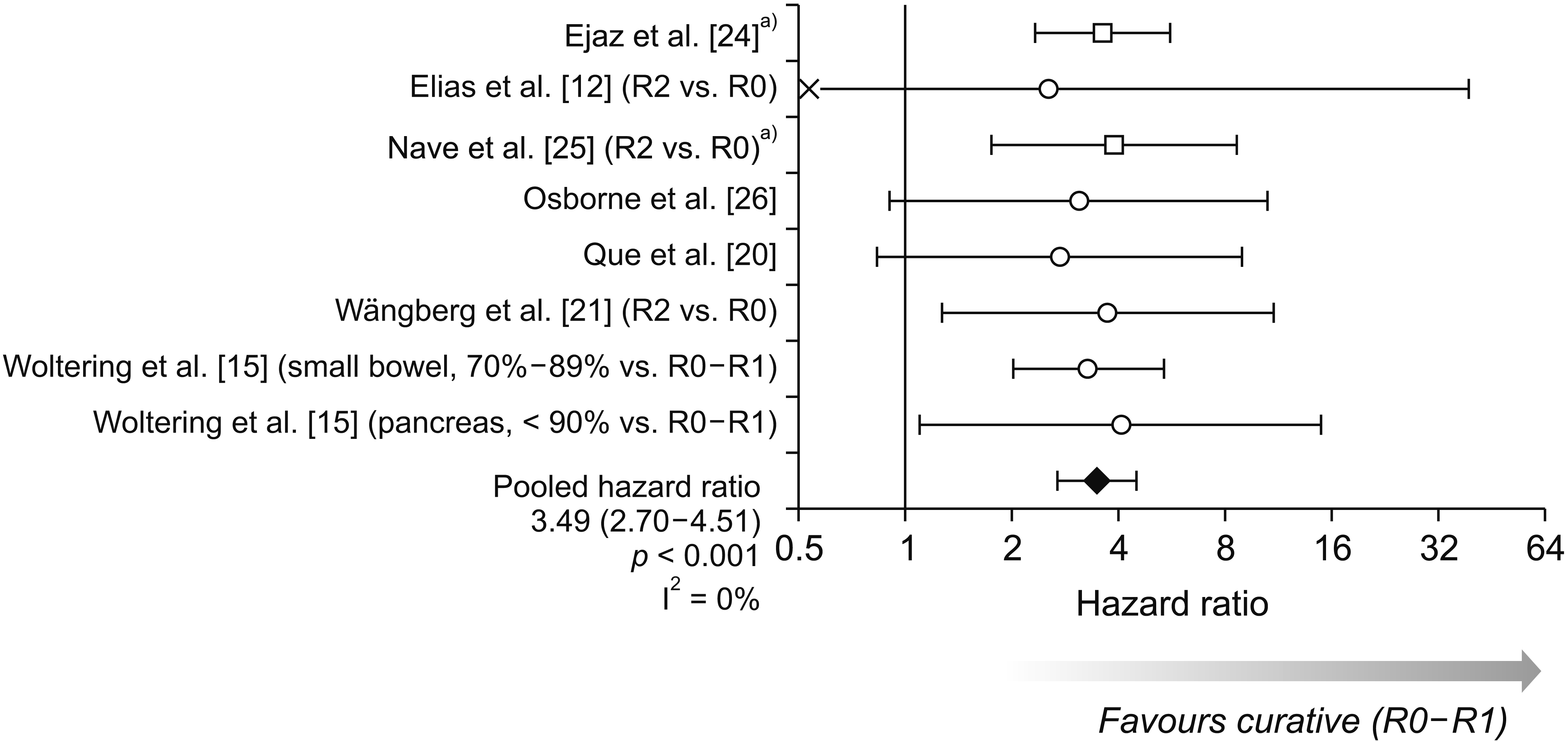
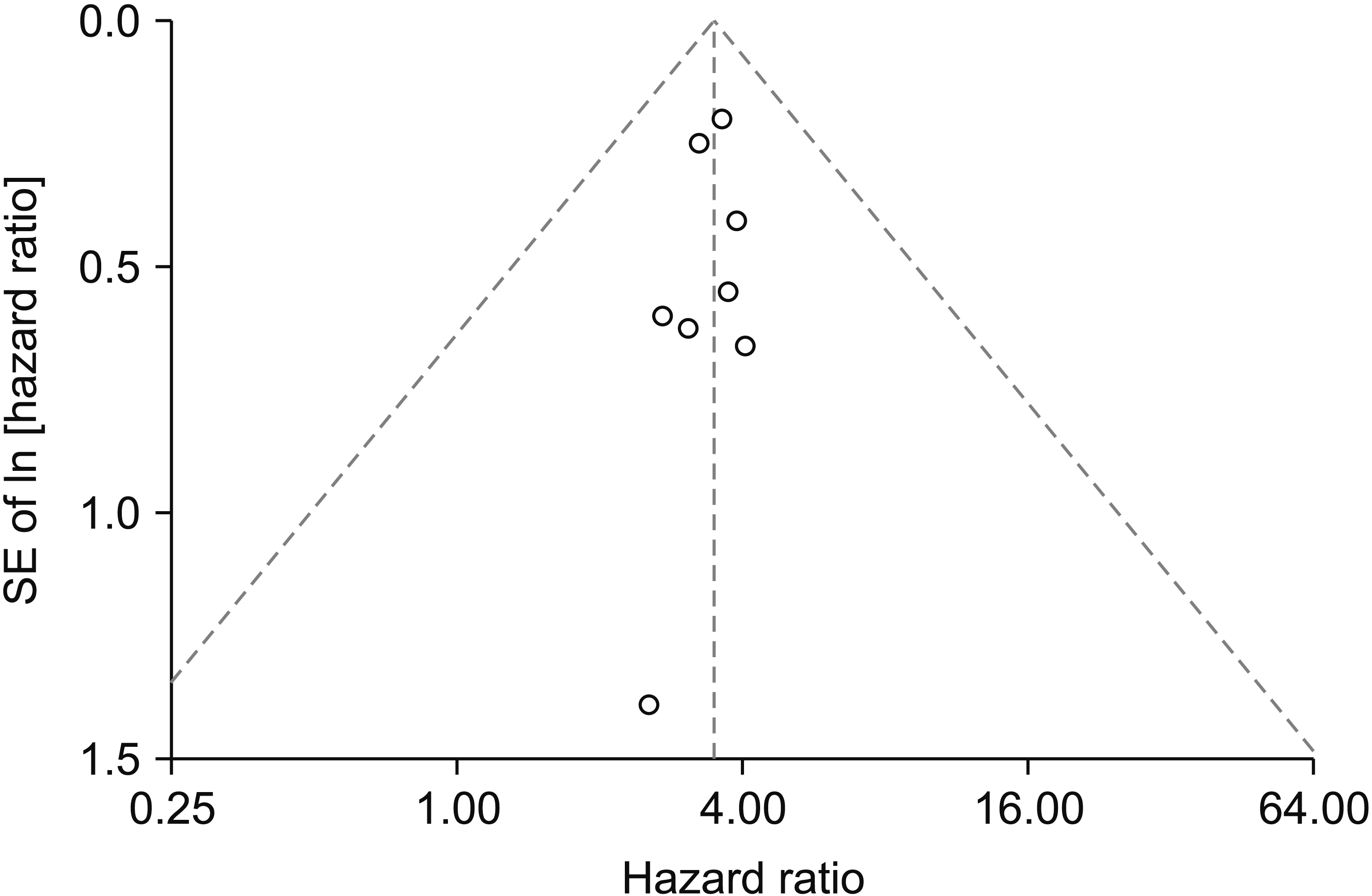
Fig. 3
NETLM debulking strategies
Table 3
| Author | Debulking | Five-year OS (median) | Five-year PFS (median) | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Group A vs. B | Group A | Group B | p-value | Group A | Group B | p-value | |||
| ≥ 90% vs. < 90% debulking | |||||||||
| Maxwell et al. [13] (small bowel) | ≥ 90% vs. < 90% | Not reached | 9.1 yr | 0.46 | 3.8 yr | 1.6 yr | 0.005* | ||
| Maxwell et al. [13] (pancreas) | ≥ 90% vs. < 90% | Not reached | 6.1 yr | 0.14 | 4.4 yr | 1.3 yr | 0.05* | ||
| Woltering et al. [15] (small bowel) | 90%–98% vs. 70%–89% | 87% (22.9 yr) | 89% (12.3 yr) | NS | - | - | - | ||
| Woltering et al. [15] (pancreas) | 90%–98% vs. < 90% | 68% (6.7 yr) | 56% (6.3 yr) | 0.015* | - | - | - | ||
| Morgan et al. [14] | ≥ 90% vs. 70%–89% | NR | NR | 0.29 | NRa) | NRa) | 0.75a) | ||
| Graff-Baker et al. [22] | 90%–99% vs. 70%–89% | - | - | - | NRa) | NRa) | 0.74a,b) | ||
| Scott et al. [16] | > 90% vs. 70%–90% | Not reached | 11.1 yr | 0.61 | 4.7 yr | 1.7 yr | < 0.01* | ||
| ≥ 70% vs. < 70% debulking | |||||||||
| Maxwell et al. [13] (small bowel) | ≥ 70% vs. < 70% | Not reached | 9.1 yr | 0.18 | 3.2 yr | 1.7 yr | 0.005* | ||
| Maxwell et al. [13] (pancreas) | ≥ 70% vs. < 70% | Not reached | 1.7 yr | 0.001* | 3.0 yr | 0.5 yr | < 0.001* | ||
| Woltering et al. [15] (small bowel) | 70%–89% vs. < 70% | 89% (12.3 yr) | 64% (7.4 yr) | NR | - | - | - | ||
| Scott et al. [16] | ≥ 70% vs. < 70% | 11.2 yr | 3.1 yr | < 0.001* | 1.7 yrc) | 0.9 yr | < 0.001* | ||




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download