Abstract
Backgrounds/Aims
In moderate and high-surgical risk patients with acute cholecystitis, studies comparing percutaneous cholecystostomy (PC) vs. endoscopic transpapillary gallbladder stenting (ETGS) vs. endoscopic ultrasound-guided transmural gallbladder stenting (EUGS) are limited. Thus, the aim of this study was to compare efficacy and recurrence of cholecystitis after PC, ETGS, or EUGS during follow-up.
Methods
We reviewed 143 moderate and high-surgical risk patients with acute cholecystitis with or without concomitant common bile duct stones who underwent PC, ETGS, or EUGS at our hospital. Technical success rate (TSR), clinical success rate (CSR), and recurrence were compared.
Results
TSR in PC or EUGS group was higher than that in the ETGS group for those with concomitant common bile duct stones (100% vs. 100% vs. 73.2%; p = 0.07) and for those without concomitant common bile duct stones (100% vs. 100% vs. 77.3%; p < 0.001). CSR in ETGS or EUGS group was higher than that in the PC group for those with concomitant common bile duct stones (96.2% vs. 100% vs. 87.5%; p = 0.41) and for those without concomitant common bile duct stones (94.1% vs. 100% vs. 63.0%; p = 0.006). Using Kaplan–Meier analysis, the overall recurrent risk was the highest in the PC group (p = 0.004).
Conclusions
In moderate and high-surgical risk patients with acute cholecystitis, EUGS provides significantly higher CSR with comparable TSR to PC. Thus, ETGS should be the first choice in those with concomitant common bile duct stones. Among the three patient groups, those who received PC had the highest rate of recurrence.
Although percutaneous cholecystostomy (PC) can be performed as an alternative treatment for gallbladder drainage in patients with acute cholecystitis who are not surgical candidates [1,2], adverse events related to PC tube have been demonstrated in up to 19% cases requiring a high rate of reintervention [3]. To obviate the need for PC, endoscopic transpapillary gallbladder stenting (ETGS) using endoscopic retrograde cholangiopancreatography (ERCP) and endoscopic ultrasound-guided transmural gallbladder stenting (EUGS) can be performed for gallbladder drainage [4-9].
ETGS can be a proper alternative treatment when PC and EUGS are contraindicated due to ascites, coagulopathy, or inaccessible gallbladder anatomy [10,11]. Particularly, ETGS can be performed at the same session of ERCP in acute cholecystitis patients with concomitant common bile duct stones [12]. Furthermore, the surgeon can remove a transpapillary gallbladder stent at the time of cholecystectomy. Nevertheless, previous studies have reported various technical success rates (TSR), ranging from 50% to 96% for ETGS in patients with acute cholecystitis [6,12-16]. The main limitation of ETGS is its inability to identify and access a cystic duct under fluoroscopy.
Recently, advances in therapeutic endosonography have led to the introduction of EUGS as an alternative of PC. Earlier data showed high TSR and clinical success rates (CSR) of acute cholecystitis in patients who underwent EUGS [17,18], with a procedure-related adverse event (PAE) rate of 13% [19]. However, studies comparing PC vs. ETGS vs. EUGS are limited [4,20]. Thus, the aim of this study was to evaluate the efficacy of PC vs. ETGS vs. EUGS in moderate and high-surgical risk patients having acute calculous cholecystitis with or without concomitant common bile duct stones. Recurrent cholecystitis rates in these particular patients during follow up were also determined.
Between January 2016 and May 2019, moderate and high-surgical risk patients who had acute cholecystitis with or without concomitant common bile duct stones and underwent PC or ETGS or EUGS at our institute were retrospectively reviewed. Acute cholecystitis was diagnosed according to the Tokyo Guideline 2018 [21]. The evidence of suspected common bile duct stones was based on (1) common bile duct stones on transabdominal ultrasound or any cross-sectional imaging studies or (2) serum total bilirubin > 4 mg/dL with common bile duct dilatation on imaging studies [22]. Contraindications of laparoscopic cholecystectomy for acute cholecystitis were classified as absolute (inability to tolerate general anesthesia or laparotomy, uncorrected coagulopathy, and suspected gallbladder cancer) or relative (advanced cirrhosis/liver failure, unstable medical conditions, previous abdominal operations which prevent safe abdominal access, and cholecysto-enteric fistula). During the study period, based on our real practice in moderate and high-surgical risk patients with acute calculous cholecystitis, indications for gallbladder drainage included patients who were poor surgical candidates due to medical conditions with clinical status stable enough for gallbladder drainage at pre-operative evaluation by medical and surgical team and willing to comply with gallbladder drainage procedures. Patients with advanced malignancy-related to acute cholecystitis, those with concomitant septic cholangitis with unstable medical conditions, and patients who refused gallbladder drainage were excluded from this study. They only received medical treatment. Nevertheless, in those with advanced malignancy related to acute cholecystitis, EUGS and PC were offered for gallbladder drainage when medical treatment was unsuccessful. The decision to have patients undergo PC or ETGS or EUGS was made by an attending gastroenterologist, a surgeon, and an interventional radiologist depending on the presence of common bile duct stones, the evidence of complete cystic duct obstruction at ERCP, potential surgical candidate after improvement of acute cholecystitis-related septic condition, the availability of endoscopic gallbladder drainage, and patient’s risk for sedation (Fig. 1). We defined recurrent cholecystitis as recurrence of clinical symptoms and imaging findings of acute cholecystitis. Patients were censored on their last visit date or the date of cholecystectomy. The study protocol was approved by Faculty of Medicine, Chulalongkorn University Institutional Review Board (IRB No. 408/62).
We defined moderate to high-surgical risk patients as those having Charlson’s comorbidity index ≥ 3. Technical success was defined when stent position was confirmed endoscopically and radiographically. Clinical success was defined when complete resolution of clinical symptoms was achieved within 72 hours. We defined PAE as any adverse events related to procedures that occurred within two weeks after the procedure.
ETGS was attempted when concomitant common bile duct stones were present or if the patient was still a potential surgical candidate after improvement of acute cholecystitis-related septic condition. After biliary sphincterotomy was done, common bile duct stone extraction was successfully performed prior to ETGS attempts. Cystic duct was cannulated under fluoroscopic guidance using a bendable-tip catheter with either a 0.899-mm (0.035-inch) or a 0.635-mm (0.025-inch) guidewire (Fig. 2). If cystic duct cannulation was not achieved within 10 minutes under fluoroscopy or if the absence of filling of the cystic duct was noted, a digital cholangioscope (SpyGlass DS Direct Visualization System; Boston Scientific, Marlborough, MA, USA) was used for identifying the cystic duct take-off under direct visualization. After the guidewire was coiled in the gallbladder, a 7-Fr 15-cm double pigtail plastic stent was successfully placed over the guidewire.
EUGS was primarily performed when complete cystic duct obstruction was observed by cholangiogram during primary ERCP or if the patient was not a potential surgical candidate after the improvement of acute cholecystitis-related septic condition. Using a 19-guage needle with Doppler flow guidance, the initial puncture was performed via either a transduodenal or a transgastric approach to access the gallbladder (Fig. 3). A 6-Fr cystotome and a 6-mm balloon were placed over the 0.635-mm (0.025-inch) guidewire to create and dilate the fistula tract between the gallbladder and the duodenum, respectively. Eventually, a non-electrocautery-enhanced lumen-apposing metal stent (LAMS) (10- or 16-mm in diameter, Niti-S Spaxus; Taewoong Medical, Gimpo, Korea) or a fully covered self-expandable metallic stent (FCSEMS) (6-cm in length, Niti-S Biliary stent; Taewoong Medical) was successfully placed.
PC was performed by interventional radiologists using local anesthesia and/or mild intravenous sedation when moderate to high-surgical risk patients had a variety of risk factors that might affect the use of conscious sedation during the endoscopic procedure such as septic conditions not amendable for sedation, heart disease, pulmonary disease, sleep apnea, or if endoscopic gallbladder drainage was not available at that time. Under transabdominal ultrasound and fluoroscopic guidance, an 18-guage needle was punctured into the gallbladder lumen. Following the tract dilation, an 8- to 10-Fr pigtail drainage catheter was inserted over the guidewire into the gallbladder.
Following successful gallbladder drainage, elective cholecystectomy was offered to patients with improvement of clinical status. For those with poor clinical status, multiple severe comorbidities, and refusal of surgery, the stent was placed permanently. In patients receiving PC, the PC tube was re-evaluated to confirm unclogging at four weeks after the procedure by the interventional radiologist and then left in situ with the bag. In patients receiving EUGS, once the gallbladder was cleared, LAMS or FCSEMS was removed at two weeks after initial placement and a 10-Fr 5-cm plastic stent was then replaced to maintain a permanent fistula tract. After discharge, all patients were followed up as outpatients every three to six months.
To compare among the three groups, we used comparative statistics including Fisher’s exact test for proportions and Kruskal–Wallis equality test or analysis of variance for continuous variables. Differences across groups were considered significant if the p-value was less than 0.05. Recurrent rate was defined as a time-to-event outcome using the Kaplan–Meier method. A log-rank test was applied for comparing risks of recurrence. IBM SPSS version 23 for Windows software (IBM Corp., Armonk, NY, USA) was used for statistical analyses.
Of 171 moderate and high-surgical risk patients with acute cholecystitis, 5 patients with advanced malignancy-related to acute cholecystitis, 6 patients with concomitant severe sepsis who refused gallbladder drainage, and 17 patients with unstable medical conditions to undergo any interventions were excluded. They received medical treatment only. Finally, a total of 143 moderate and high-surgical risk patients with acute calculous cholecystitis were enrolled in this study (Fig. 4).
Of 93 patients receiving ETGS, 69 patients (74.2%) had successful ETGS either by fluoroscopic (n = 49) or digital cholangioscopic guidance (n = 20). Of 24 patients who failed ETGS, 7 patients subsequently received successful EUGS, 12 patients continued to have medical treatment alone, and 5 patients had cholecystectomy at the same admission. Of 30 patients having cystic duct stones with identifiable cystic duct, 20 patients had successful ETGS whereas 10 patients had failed ETGS due to small caliber and tortuous cystic duct.
Of 22 patients receiving EUGS, 15 patients who had EUGS after ERCP showed complete cystic duct obstruction by cholangiogram (n = 8) and complicated cholecystitis on CT scan (n = 7), whereas 7 patients had subsequent EUGS following unsuccessful ETGS. The overall TSR of patients having EUGS was 22/22 (100%).
All 35 patients with acute cholecystitis received one session of PC which was technically successful.
In acute cholecystitis patients with or without concomitant common bile duct stones, overall TSRs were similar between PC and EUGS groups, but higher than the TSR of the ETGS group (35/35 [100%] vs. 22/22 [100%] vs. 69/93 [74.2%]; p < 0.001). Overall CSR in ETGS and EUGS groups were comparable, but higher than the CSR in the PC group (66/69 [95.7%] vs. 22/22 [100%] vs. 24/35 [68.6%]; p < 0.001). The length of stay of the PC group was longer than that in the ETGS group or the EUGS group (14 vs. 4. vs. 7 days; p < 0.001).
In acute cholecystitis patients with concomitant common bile duct stones (n = 86), TSR were similar between PC and EUGS groups and higher, but not significantly higher, than the TSR of the ETGS group (8/8 [100%] vs. 10/10 [100%] vs. 52/71 [73.2%]; p = 0.07). CSRs in ETGS and EUGS groups were comparable and higher, but not significantly higher, than the CSR in the PC group (50/52 [96.2%] vs. 10/10 [100%] vs. 7/8 [87.5%]; p = 0.41). One patient with clinical failure in the PC group who could not tolerate further intervention expired from uncontrolled infection.
In acute cholecystitis patients without concomitant common bile duct stones (n = 57), TSR were similar between PC and EUGS groups but significantly higher than the TSR of the ETGS group (27/27 [100%] vs. 12/12 [100%] vs. 17/22 [77.3%]; p < 0.001). CSRs of ETGS and EUGS groups were comparable but significantly higher than the CSR of the PC group (16/17 [94.1%] vs. 12/12 [100%] vs. 17/27 [63.0%]; p = 0.006). All 10 patients with clinical failure in the PC group could not tolerate further intervention. They expired from uncontrolled infection.
PAEs were not significantly different among ETGS, EUGS, and PC for acute cholecystitis patients with concomitant common bile duct stones (10.3% vs. 10.0% vs. 0%; p = 0.55) or for those without concomitant common bile duct stones (11.1% vs. 0% vs. 0%, p = 0.93) (Table 1). In acute cholecystitis patients with or without concomitant common bile duct stones (n = 143), PAEs included mild pancreatitis (n = 1), post sphincterotomy bleeding which spontaneously stopped (n = 3), guidewire perforation not needing further intervention (n = 4), and a higher spike of temperature which was successfully treated by antibiotics (n = 1). In the EUGS group (n = 22), minimal bile leak was observed based on contrast leakage under fluoroscopy during the procedure (n = 8). However, only one was considered as PAE by having a higher spike of temperature. It was resolved by conservative treatment.
In acute cholecystitis patients with or without concomitant common bile duct stones (n = 143), the overall recurrent rate was higher in the PC group than that in the ETGS or EUGS group (17.1% vs. 4.6% vs. 4.5%; p = 0.05). Using Kaplan–Meier analysis (Fig. 5), the overall risk of recurrence was the highest in the PC group (p = 0.004) among the three groups during a median follow up of six months. Elective cholecystectomy was performed in 59.3% and 37.1% of patients in ETGS and PC groups, respectively. In the EUGS group, none received cholecystectomy due to severe comorbidities. For those receiving ETGS or PC who eventually underwent cholecystectomy (n = 64), there was no recurrence. For those receiving ETGS, EUGS, or PC whose drainage was left permanently without cholecystectomy (n = 89), the recurrent rate was higher in the PC group than in ETGS and EUGS groups (27.1% vs. 9.3% vs. 4.8%; p = 0.04). Of 143 patients, 19 (13.3%) (ETGS, 10; EUGS, 8; PC, 1) were followed for more than one year. Among those 19 patients, one patient in the ETGS group developed recurrent cholecystitis due to stent occlusion. Finally, two patients underwent elective cholecystectomy and two patients died from myocardial infarction.
In acute cholecystitis patients with concomitant common bile duct stones, the cause of recurrence was stent occlusion which occurred in 4 and 3 patients who did not undergo cholecystectomy in the PC group and the ETGS group, respectively. They all received stent exchange. Ten patients were lost to follow up.
In acute cholecystitis patients without concomitant common bile duct stones, one patient developed recurrent cholecystitis at 4 months after LAMS placement and was found to have LAMS migration which required subsequently repeat EUGS. Six patients were lost to follow up.
Recently, a large retrospective multicenter study from the United States (US) (n = 372) compared PC (n = 146) vs. ETGS (n = 124) vs. EUGS (n = 102) and showed that CSR of PC was comparable to that of EUGS but higher than that of ETGS (98% vs. 90% vs. 80%, p < 0.001) [4]. However, main limitations of these data included multiple sessions to achieve CSR in the PC group and only one session was allowed for an endoscopic drainage. In addition, the US multicenter study lacked patient-selection criteria for gallbladder drainage in each group [4]. Unlike previous results [4], we provided a decision tree to have patients undergo ETGS, EUGS, or PC during the study period. The decision tree was mainly based on the presence of common bile duct stones, the evidence of complete cystic duct obstruction at ERCP, and potential surgical candidate after the improvement of acute cholecystitis related septic condition. Our data showed a lower CSR in the PC group compared to those in EUGS and ETGS groups. In our study, PC was performed in a single session. The PC tube was then placed until ultimate cholecystectomy was done if no recurrence. All patients with clinical failure in the PC group expired from severe medical conditions at presentation, including septic shock with multiorgan failure (n = 8) and advanced lung cancer (n = 2), which might have resulted in patient-selection bias because more patients with poorer medical condition in this retrospective series were included in the PC group. Likewise, another recent meta-analysis of 22 studies comparing PC vs. EUGS vs. ETGS has found that the CSR in the PC group is lower than that of the EUGS group (89.3% vs. 96.7%; p = 0.001), but comparable to that of the ETGS group (89.3% vs. 88.1%; p = 0.59) [20]. Of note, the PC in that meta-analysis was mainly performed in a single session.
Two retrospective studies have compared ETGS vs. EUGS and demonstrated higher TSR in the EUGS group than in the ETGS group (99.3% vs. 86.6%, p < 0.01; and 97.5% vs. 84.2%, p = 0.06) [5,7]. However, these two retrospective studies did not mention the presence of common bile duct stones in patients undergoing ETGS. Although these studies, including ours, suggest that EUGS is better treatment than ETGS regarding TSR and CSR, we suggest ETGS if patients have concomitant common bile duct stones which could be performed in the same session of ERCP with common bile duct stone removal. Those with planned cholecystectomy are also candidates for ETGS because it can preserve gallbladder anatomy and surrounding structures. However, in a EUGS patient, the surgeon must close the fistula between the gallbladder and gastrointestinal tract [23].
Our previous publication showed a lower overall TSR than that in the study of Yoshida et al. [24] despite cholangioscopy assistance (75% vs. 94%) [12]. However, our result is still inline of TSRs ranging from 50% to 90% from other studies [6,12-16,24]. We had more patients with inaccessible cystic duct anatomy (small caliber and tortuous cystic duct), cystic duct obstruction from impact cystic duct stones, and the presence of posterior cystic duct insertion, which might have resulted in technical difficulty for cystic duct cannulation and guidewire placement into the gallbladder.
In our study, 51 patients (59.3%) with ETGS and 13 patients (37.1%) with PC further underwent cholecystectomy without surgical complications, with a median time from gallbladder drainage to cholecystectomy of 130 days and 72 days, respectively. In a multi-center retrospective study of laparoscopic cholecystectomy after PC and endoscopic naso gallbladder drainage for acute cholecystitis (n = 347) reported by Tomimaru et al. [25], the time for cholecystectomy in their series were within 2 weeks and after 1 month, respectively, with good surgical outcomes. In a study of cholecystectomy after EUGS (n = 13) and PC (n = 21) reported by Saumoy et al. [26], all patients had technically successful with the mean time from gallbladder drainage to cholecystectomy of 156 days and 80 days, respectively. Based on these available data, the optimal time for cholecystectomy should be performed within three months. The earliest could be within two weeks after gallbladder drainage in those with severe cholecystitis who underwent ETGS and PC. However, in those who receive EUGS, an elective cholecystectomy could be performed at a later period. We recommend at least after one month for the fistula between the gallbladder and the gastrointestinal lumen becomes mature before considering surgery.
Similar to the US multicenter study comparing PAE among ETGS, EUGS, and PC groups (7% vs. 12% vs. 4%; p = 0.07) [4] and the meta-analysis performed by Mohan et al. [20] (9.6% vs. 12.4% vs. 15.1%, p = 0.12), we found that PAEs were not significantly different among ETGS, EUGS, and PC groups. On the contrary, the study by Higa et al. [5], the US multicenter study [4], and the meta-analysis by Mohan et al. [20] showed higher rates of PAE in the EUGS group than in the ETGS group (17.9% vs. 9.4%, p = 0.18; 11.8% vs. 7.2%, p = 0.07; and 12.4% vs. 9.6%, p = 0.32, respectively), although the difference did not reach statistical significance. The study by Higa et al. [5] and the US multicenter study reported death and emergency surgery due to PAE after LAMS placement [4]. In the meta-analysis by Mohan et al. [20] comparing among ETGS, EUGS, and PC, bleeding (1.9% vs. 4.3% vs. 2%; p = 0.02) and perforation (2% vs. 3.7% vs. 2%; p = 0.04) were higher in the EUGS group than in ETGS and PC groups. Potential causes of bias could be due to variations in causes of cholecystitis (calculous or advanced malignancy related acalculous cholecystitis) and the early phase of learning in EUGS for LAMS deployment by inexperienced endoscopists [27]. In a multicenter registry on EUGS mainly from Asia-Pacific countries, more unplanned procedural events occurred in EUGS performed by endoscopists with experience of < 25 EUGS procedures than by those with experience of ≥ 25 EUGS procedures (13.5% vs. 5.8%; p = 0.012) [27]. In our series, we experienced mis-deployment of LAMS in our initial 3 cases receiving EUGS. Another FCSEMS was subsequently placed as stent-in-stent to rescue.
In addition to patients who underwent elective cholecystectomy (44.7%), approximately one-third of patients were lost to follow up with death due to other causes such as congestive heart failure (n = 12), myocardial infarction (n = 8), bacterial pneumonia (n = 5), end-stage liver disease (n = 6), and advanced malignancy (n = 4), which might have resulted in a short median follow-up period in our study. Similar to the US multicenter study comparing recurrent rates among PC, ETGS, and EUGS (20% vs. 5% vs. 2%; p < 0.001) [4] and the meta-analysis performed by Mohan et al. [20] (10.8% vs. 4.6% vs. 4.2%, p = 0.001), the recurrent rate in the PC group was significantly higher than that in the ETGS or EUGS group in our study. Thus, PC should not be the definitive treatment in patients with acute cholecystitis who are not surgical candidates because stent occlusion could develop in the long term.
Limitations of our study include its retrospective design and the possibility of selection bias for gallbladder draining techniques by endoscopists. Nevertheless, our study demonstrated results from a practical standpoint because we selected procedures for patients based on many practical parameters including surgical risk, concomitant common bile duct stones, and cystic duct anatomy (Fig. 1). In our opinion, a large prospective study comparing PC vs. ETGS vs. EUGS in a controlled environment with similar settings of patients with acute cholecystitis is difficult. It would be better to customize the choice of drainage based on clinical presentation, gallbladder anatomy, surgical candidacy, and long-term treatment plans.
In conclusion, although EUGS and PC are comparable in terms of TSR, EUGS provides better outcomes than PC regarding CSR. The importance of this finding is that EUGS is practically more suitable for permanent gallbladder drainage in patients with acute cholecystitis who are not surgical candidates. However, ETGS and PC are better choices for those who are candidates of a future cholecystectomy. Particularly, ETGS should be the first choice for those with concomitant common bile duct stones because ERCP for common bile duct stone removal can be performed during the same session.
ACKNOWLEDGMENT
An earlier version of these data has been selected for presentation at the annual meeting of Digestive Diseases Week, 2020, Chicago, Illinois, USA.
Notes
REFERENCES
1. Iino C, Shimoyama T, Igarashi T, Aihara T, Ishii K, Sakamoto J, et al. 2018; Comparable efficacy of endoscopic transpapillary gallbladder drainage and percutaneous transhepatic gallbladder drainage in acute cholecystitis. Endosc Int Open. 6:E594–E601. DOI: 10.1055/s-0044-102091. PMID: 29744378. PMCID: PMC5940465.

2. Okamoto K, Suzuki K, Takada T, Strasberg SM, Asbun HJ, Endo I, et al. Tokyo Guidelines 2018: flowchart for the management of acute cholecystitis. J Hepatobiliary Pancreat Sci. 2018; 25:55–72. DOI: 10.1002/jhbp.516. PMID: 29045062.
3. Boules M, Haskins IN, Farias-Kovac M, Guerron AD, Schechtman D, Samotowka M, et al. 2017; What is the fate of the cholecystostomy tube following percutaneous cholecystostomy? Surg Endosc. 31:1707–1712. DOI: 10.1007/s00464-016-5161-x. PMID: 27519595.

4. Siddiqui A, Kunda R, Tyberg A, Arain MA, Noor A, Mumtaz T, et al. 2019; Three-way comparative study of endoscopic ultrasound-guided transmural gallbladder drainage using lumen-apposing metal stents versus endoscopic transpapillary drainage versus percutaneous cholecystostomy for gallbladder drainage in high-risk surgical patients with acute cholecystitis: clinical outcomes and success in an International, Multicenter Study. Surg Endosc. 33:1260–1270. DOI: 10.1007/s00464-018-6406-7. PMID: 30209610.

5. Higa JT, Sahar N, Kozarek RA, La Selva D, Larsen MC, Gan SI, et al. 2019; EUS-guided gallbladder drainage with a lumen-apposing metal stent versus endoscopic transpapillary gallbladder drainage for the treatment of acute cholecystitis (with videos). Gastrointest Endosc. 90:483–492. DOI: 10.1016/j.gie.2019.04.238. PMID: 31054909.

6. Itoi T, Coelho-Prabhu N, Baron TH. 2010; Endoscopic gallbladder drainage for management of acute cholecystitis. Gastrointest Endosc. 71:1038–1045. DOI: 10.1016/j.gie.2010.01.026. PMID: 20438890.

7. Oh D, Song TJ, Cho DH, Park DH, Seo DW, Lee SK, et al. 2019; EUS-guided cholecystostomy versus endoscopic transpapillary cholecystostomy for acute cholecystitis in high-risk surgical patients. Gastrointest Endosc. 89:289–298. DOI: 10.1016/j.gie.2018.08.052. PMID: 30213575.

8. Irani S, Ngamruengphong S, Teoh A, Will U, Nieto J, Abu Dayyeh BK, et al. 2017; Similar efficacies of endoscopic ultrasound gallbladder drainage with a lumen-apposing metal stent versus percutaneous transhepatic gallbladder drainage for acute cholecystitis. Clin Gastroenterol Hepatol. 15:738–745. DOI: 10.1016/j.cgh.2016.12.021. PMID: 28043931.

9. Anderloni A, Buda A, Vieceli F, Khashab MA, Hassan C, Repici A. 2016; Endoscopic ultrasound-guided transmural stenting for gallbladder drainage in high-risk patients with acute cholecystitis: a systematic review and pooled analysis. Surg Endosc. 30:5200–5208. DOI: 10.1007/s00464-016-4894-x. PMID: 27059975.

10. Salameh H, DiMaio CJ. 2019; Endoscopic retrograde cholangiopancreatography and endoscopic ultrasound-guided gallbladder drainage. Gastrointest Endosc Clin N Am. 29:293–310. DOI: 10.1016/j.giec.2018.12.002. PMID: 30846154.

11. Johlin FC Jr, Neil GA. 1993; Drainage of the gallbladder in patients with acute acalculous cholecystitis by transpapillary endoscopic cholecystotomy. Gastrointest Endosc. 39:645–651. DOI: 10.1016/S0016-5107(93)70216-3. PMID: 8224686.

12. Ridtitid W, Piyachaturawat P, Teeratorn N, Angsuwatcharakon P, Kongkam P, Rerknimitr R. 2020; Single-operator peroral cholangioscopy cystic duct cannulation for transpapillary gallbladder stent placement in patients with acute cholecystitis at moderate to high surgical risk (with videos). Gastrointest Endosc. 92:634–644. DOI: 10.1016/j.gie.2020.03.3866. PMID: 32330504.

13. McCarthy ST, Tujios S, Fontana RJ, Rahnama-Moghadam S, Elmunzer BJ, Kwon RS, et al. 2015; Endoscopic transpapillary gallbladder stent placement is safe and effective in high-risk patients without cirrhosis. Dig Dis Sci. 60:2516–2522. DOI: 10.1007/s10620-014-3371-4. PMID: 25287001.

14. Maekawa S, Nomura R, Murase T, Ann Y, Oeholm M, Harada M. 2013; Endoscopic gallbladder stenting for acute cholecystitis: a retrospective study of 46 elderly patients aged 65 years or older. BMC Gastroenterol. 13:65. DOI: 10.1186/1471-230X-13-65. PMID: 23586815. PMCID: PMC3675408.
15. Lee TH, Park DH, Lee SS, Seo DW, Park SH, Lee SK, et al. 2011; Outcomes of endoscopic transpapillary gallbladder stenting for symptomatic gallbladder diseases: a multicenter prospective follow-up study. Endoscopy. 43:702–708. DOI: 10.1055/s-0030-1256226. PMID: 21425042.

16. Kjaer DW, Kruse A, Funch-Jensen P. 2007; Endoscopic gallbladder drainage of patients with acute cholecystitis. Endoscopy. 39:304–308. DOI: 10.1055/s-2007-966335. PMID: 17427067.

17. Irani S, Baron TH, Grimm IS, Khashab MA. 2015; EUS-guided gallbladder drainage with a lumen-apposing metal stent (with video). Gastrointest Endosc. 82:1110–1115. DOI: 10.1016/j.gie.2015.05.045. PMID: 26142558.

18. Khan MA, Atiq O, Kubiliun N, Ali B, Kamal F, Nollan R, et al. 2017; Efficacy and safety of endoscopic gallbladder drainage in acute cholecystitis: is it better than percutaneous gallbladder drainage? Gastrointest Endosc. 85:76–87.e3. DOI: 10.1016/j.gie.2016.06.032. PMID: 27343412.

19. Mohan BP, Asokkumar R, Shakhatreh M, Garg R, Ponnada S, Navaneethan U, et al. 2019; Adverse events with lumen-apposing metal stents in endoscopic gallbladder drainage: a systematic review and meta-analysis. Endosc Ultrasound. 8:241–248. DOI: 10.4103/eus.eus_63_18. PMID: 31115387. PMCID: PMC6714485.

20. Mohan BP, Khan SR, Trakroo S, Ponnada S, Jayaraj M, Asokkumar R, et al. 2020; Endoscopic ultrasound-guided gallbladder drainage, transpapillary drainage, or percutaneous drainage in high risk acute cholecystitis patients: a systematic review and comparative meta-analysis. Endoscopy. 52:96–106. DOI: 10.1055/a-1020-3932. PMID: 31645067.

21. Yokoe M, Hata J, Takada T, Strasberg SM, Asbun HJ, Wakabayashi G, et al. Tokyo Guidelines 2018: diagnostic criteria and severity grading of acute cholecystitis (with videos). J Hepatobiliary Pancreat Sci. 2018; 25:41–54. DOI: 10.1002/jhbp.515. PMID: 29032636.
22. Buxbaum JL, Abbas Fehmi SM, Sultan S, Fishman DS, Qumseya BJ, Cortessis VK, et al. 2019; ASGE guideline on the role of endoscopy in the evaluation and management of choledocholithiasis. Gastrointest Endosc. 89:1075–1105.e15. DOI: 10.1016/j.gie.2018.10.001. PMID: 30979521. PMCID: PMC8594622.

23. Rerknimitr R, Pham KC. 2020; Practical approaches for high-risk surgical patients with acute cholecystitis: the percutaneous approach versus endoscopic alternatives. Clin Endosc. 53:678–685. DOI: 10.5946/ce.2019.186. PMID: 31914724. PMCID: PMC7719420.

24. Yoshida M, Naitoh I, Hayashi K, Jinno N, Hori Y, Natsume M, et al. 2021; Four-step classification of endoscopic transpapillary gallbladder drainage and the practical efficacy of cholangioscopic assistance. Gut Liver. 15:476–485. DOI: 10.5009/gnl20238. PMID: 33402544. PMCID: PMC8129659.

25. Tomimaru Y, Fukuchi N, Yokoyama S, Mori T, Tanemura M, Sakai K, et al. 2020; Optimal timing of laparoscopic cholecystectomy after gallbladder drainage for acute cholecystitis: a multi-institutional retrospective study. J Hepatobiliary Pancreat Sci. 27:451–460. DOI: 10.1002/jhbp.768. PMID: 32460406.

26. Saumoy M, Tyberg A, Brown E, Eachempati SR, Lieberman M, Afaneh C, et al. 2019; Successful cholecystectomy after endoscopic ultrasound gallbladder drainage compared with percutaneous cholecystostomy, can it be done? J Clin Gastroenterol. 53:231–235. DOI: 10.1097/MCG.0000000000001036. PMID: 29697498.

27. Teoh AY, Perez-Miranda M, Kunda R, Lee SS, Irani S, Yeaton P, et al. 2019; Outcomes of an international multicenter registry on EUS-guided gallbladder drainage in patients at high risk for cholecystectomy. Endosc Int Open. 7:E964–E973. DOI: 10.1055/a-0915-2098. PMID: 31367676. PMCID: PMC6656552.

Fig. 1
Decision tree to have patients undergo ETGS, EUGS, or PC during the study period. CBD, common bile duct; ERCP, endoscopic retrograde cholangiopancreatography; ETGS, endoscopic transpapillary gallbladder stenting; EUGS, endoscopic ultrasound-guided transmural gallbladder stenting; PC, percutaneous cholecystostomy. a)Patients were admitted for acute cholecystitis with sepsis during weekend when emergency ETGS or EUGS was not available.
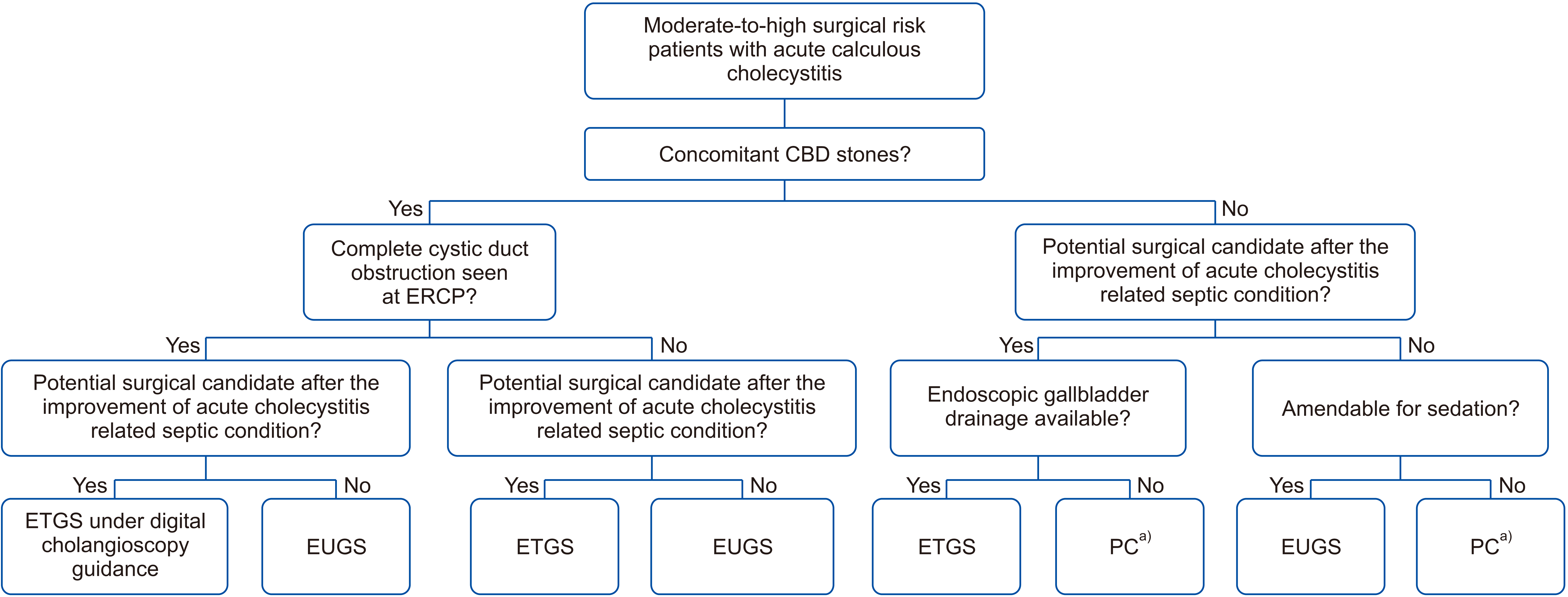
Fig. 2
Endoscopic transpapillary gallbladder stenting technique. (A) Cystic duct cannulation; (B) gallbladder guidewire placement; (C) gallbladder stent placement.
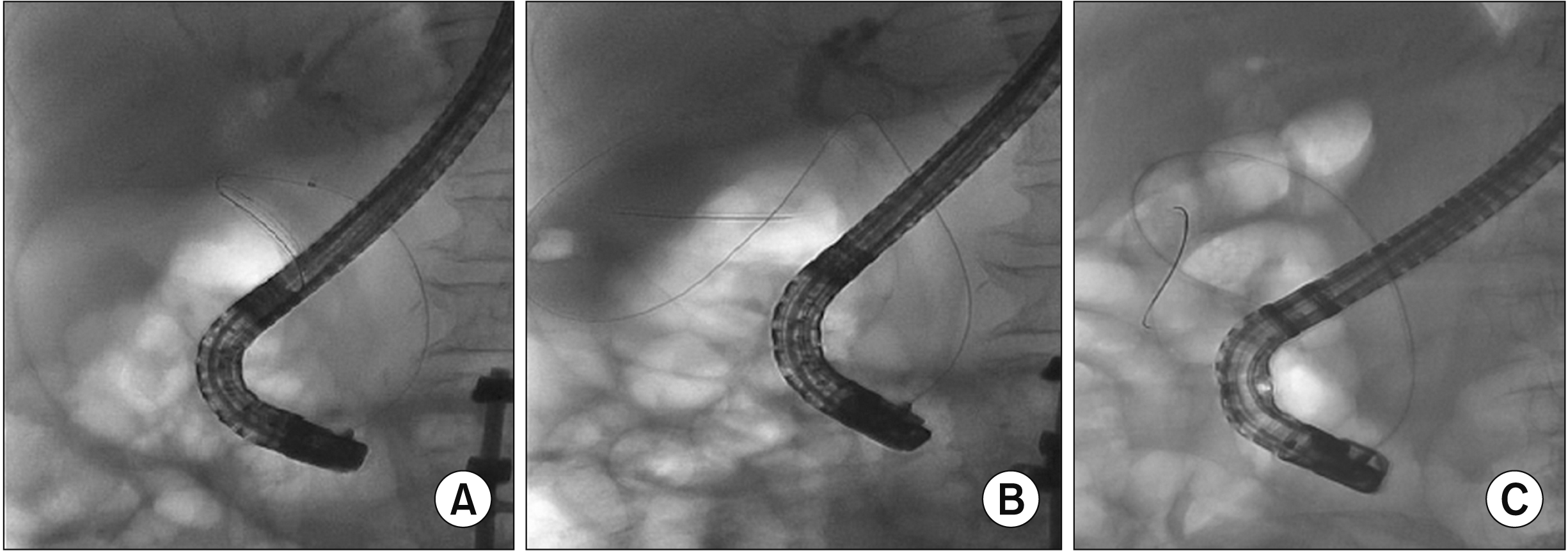
Fig. 3
Endoscopic ultrasound-guided transmural gallbladder stenting technique. (A) Contrast injection into gallbladder; (B) guidewire looping in the gallbladder; (C) creating the fistula tract using a cystotome; (D) successful lumen-apposing metal stent (LAMS) deployment; (E) pus drainage with small gallstones through LAMS; (F) gallbladder wall inflammation seen via LAMS.
Fig. 4
Patient cohort between January 2016 and May 2019. CBD, common bile duct; ETGS, endoscopic transpapillary gallbladder stenting; EUGS, endoscopic ultrasound-guided transmural gallbladder stenting; PC, percutaneous cholecystostomy. a)Three and four patients who had unsuccessful ETGS crossed over to successful EUGS in those with and without concomitant CBD stones, respectively. b)Of 12 patients who received medical treatment only, all patients had clinical success. Two patients developed recurrence at one month and received cholecystectomy in the same admission whereas 4 patients had elective cholecystectomy without any recurrence. The remaining patients (n = 6) did not receive cholecystectomy due to their severe comorbidities with Charlson’s comorbidity index ≥ 6 (severe cardiac diseases, n = 2; advanced malignancy, n = 4). They had no recurrence during a median follow-up of 102 days (range, 84–1,448 days).
Fig. 5
Kaplan–Meier analysis demonstrating overall recurrent risk in ETGS vs. EUGS vs. PC groups after non-surgical gallbladder drainage in acute cholecystitis patients with or without concomitant common bile duct stones. PC, percutaneous cholecystostomy; ETGS, endoscopic transpapillary gallbladder stenting; EUGS, endoscopic ultrasound-guided transmural gallbladder stenting.
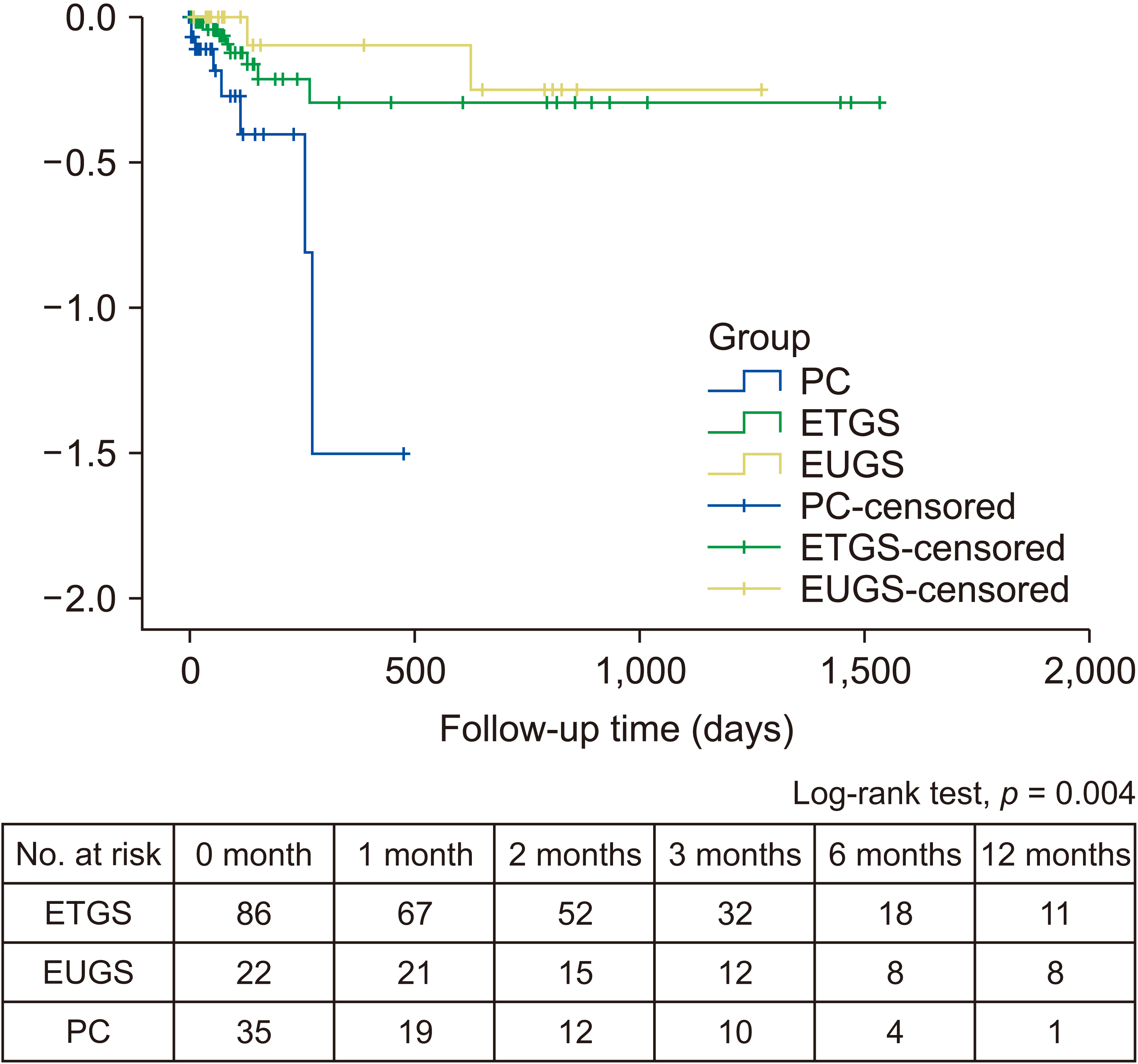
Table 1
Baseline characteristics and outcomes of patients in ETGS, EUGS, and PC groups




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download