INTRODUCTION
Olmesartan, a newly introduced angiotensin II receptor blocker (ARB), was approved in 2002 by Food and Drug Administration, and has been used in South Korea since 2005. Olmesartan-associated enteropathy (OAE) is a rare side effect of the drug. According to one systematic review, its clinical presentation includes chronic diarrhea (95%), weight loss (89%), fatigue (56%), nausea and vomiting (45%), abdominal pain (37%), and abdominal bloating (29%).
1 As it resembles coeliac disease both clinically and pathologically, OAE, along with coeliac disease, should be considered for differential diagnosis when a patient presents with such symptoms. The median years of olmesartan use before the occurrence of gastrointestinal symptoms has been reported to be 3.1 years (range 0.5-7 years) in one study, and 3.2 years in another study.
2,3 The diagnosis of OAE can be made when enteropathy with villous atrophy and a negative coeliac serology is confirmed.
4 The mainstay of treatment remains supportive care, as nearly all reported cases of OAE have shown complete recovery after the discontinuation of olmesartan.
1,2,5 We hereby report a case of OAE in a 68-year-old female patient who presented at Pusan National University Yangsan Hospital (PNUYH) with acute kidney injury (AKI) and diarrhea that had lasted for 3 months.
Go to :

CASE REPORT
A 68-year-old female, taking medication for hypertension for years, was referred to the emergency department of PNUYH for chronic diarrhea. A few weeks prior to visiting PNUYH, the patient visited a local clinic complaining of diarrhea lasting for 2 months. Basic laboratory studies including complete blood cell count, serum electrolytes, renal function test, and liver enzymes, esophagogastroduodenoscopy (EGD), and colonoscopy were done but showed unremarkable results. A week later, the patient visited a local clinic complaining of nausea, vomiting, and oliguria, and was then referred to an academic medical center. Laboratory assessments, EGD, and colonoscopy were repeated but showed no remarkable results. A week later, the patient visited both a local and an academic hospital with continuing symptoms of nausea, vomiting, and oliguria. Laboratory tests showed blood urea nitrogen (BUN), creatinine, and estimated glomerular filtration rate of 50.4 mg/dL, 7.20 mg/dL, and 6 L/min/1.73 m2, respectively. The patient was treated in an intensive care unit with 4 days of continuous renal replacement therapy (CRRT). However, severe diarrhea continued even after the complete recovery of renal function. All investigations to identify the cause of diarrhea showed normal or negative results, including tests for Clostridium difficile, viral/bacterial diarrhea, typhoid/paratyphoid fever, thyroid function, and cortisol level. An abdominal CT showed a cystic mass in the pancreas, dilatation of the extrahepatic bile duct, sludge in the gallbladder, and small peripancreatic fluid collection. In addition, repeated colonoscopy showed no abnormality. The only significant positive result relating to diarrhea was the stool calprotectin level of 1,047.0 mg/kg. As the cause of diarrhea remained unclear, the patient requested a re-evaluation.
Subsequently, the patient was referred to PNUYH and was admitted to the gastroenterology department. Repeated stool culture for infectious colitis showed negative results. To rule out rare causes of chronic diarrhea, endoscopic studies and biopsies of the whole gastrointestinal tract were performed. On EGD and colonoscopy, a single duodenal ulcer was seen and biopsies were done on the duodenum, terminal ileum, and rectum to rule out small bowel enteropathy and amyloidosis, respectively. However, no remarkable visible abnormalities were seen during these investigations. On video capsule endoscopy (VCE) of the small bowel, a diffuse loss of the villi and edematous changes in the distal duodenum and proximal jejunum were seen, indicating sprue-like enteropathy (
Fig. 1). Although all the biopsies done on EGD and colonoscopy showed non-specific inflammation, a closer look at the duodenal pathology showed a loss of villi, erosions, and chronic active inflammation of the glandular structures (
Fig. 2). A Congo-red stain for amyloidosis showed a negative result. Since coeliac disease or other sprue-like enteropathy was suspected, tests for the serum levels of anti-tissue transglutaminase (tTG) antibody IgA and total IgA were done, which were found to be negative. The patient continued to present with ongoing severe watery diarrhea that weighed over 5,000 g/day, although food and drugs including olmesartan were no longer being administered since 2 weeks before admission, to alleviate nausea, vomiting and diarrhea. After a few days of intensive care unit-based treatment, 12 mg of oral loperamide was administered daily. Subsequently, as the amount of diarrhea decreased to 1,220 g on hospital day 7, loperamide was discontinued on day 8. However, diarrhea worsened again, weighing more than 3,000 g. The same dose of oral loperamide was readministered, and the patient was discharged on hospital day 14 with the improvement in diarrheal symptoms to less than 5 times a day and a total amount under 1,000 g (
Fig. 3). Four months later, a follow-up VCE in the outpatient department showed normalized villi and the disappearance of edema on the distal duodenum and proximal jejunum (
Fig. 4).
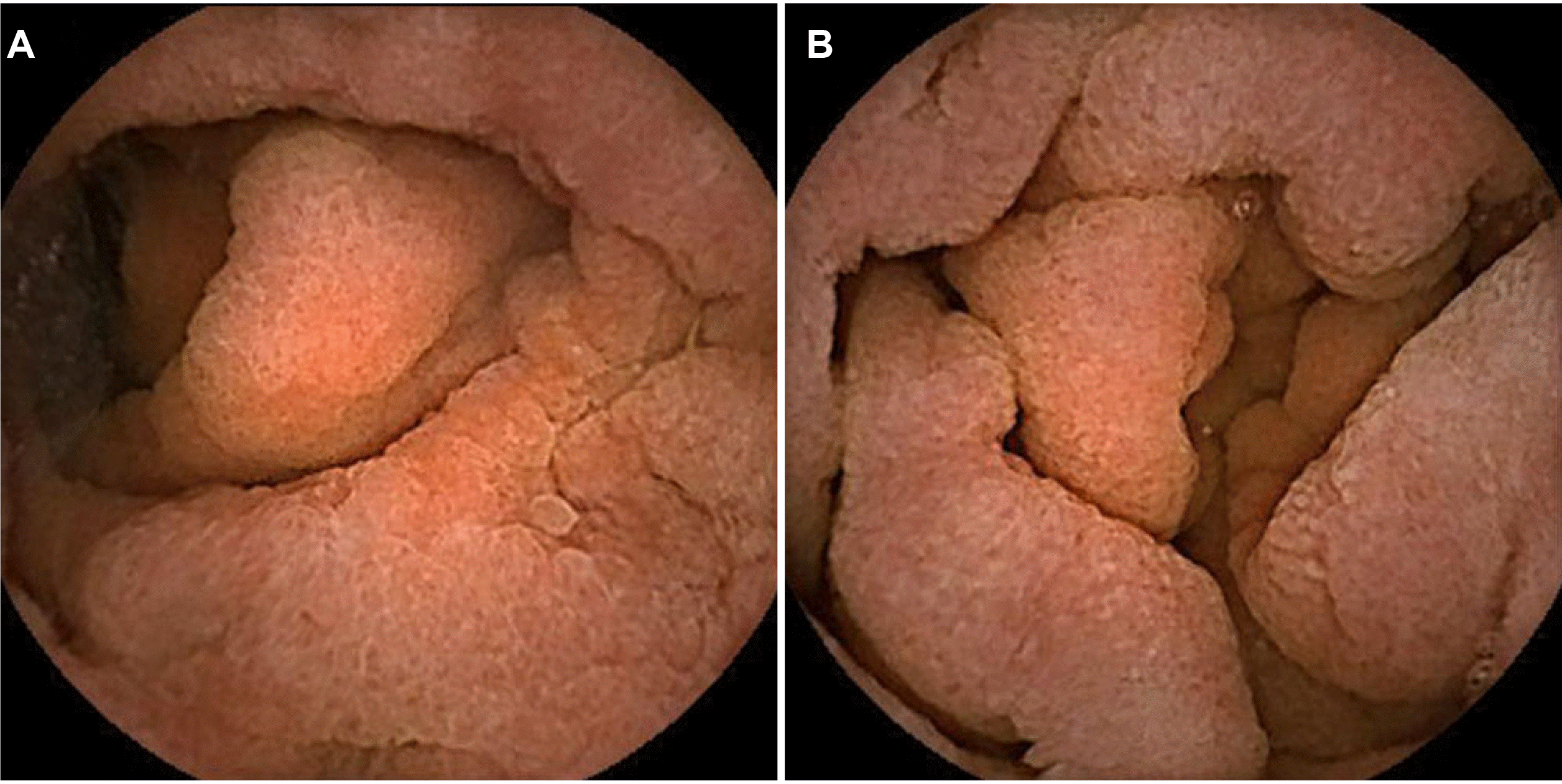 | Fig. 1Endoscopic retrograde choangiopancreatography (ERCP) at the time of initial diagnosis. ERCP reveals (A) major papilla and (B) minor papilla. 
|
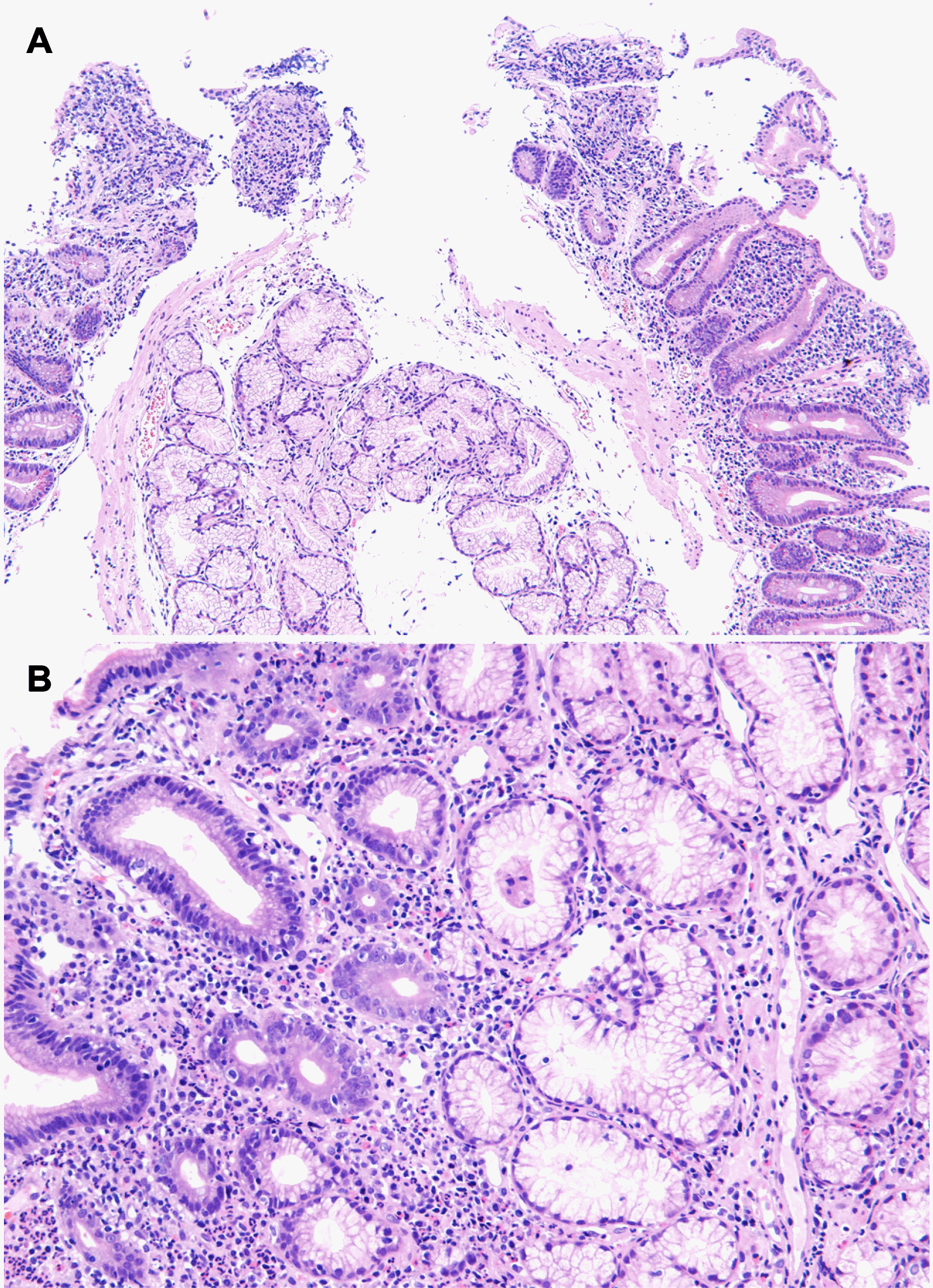 | Fig. 2Pathologic findings of the duodenum. (A) Loss of villi structures and erosion (hematoxylin and eosin [H&E], ×100) and (B) chronic active inflammation of gland structures (H&E, ×200). 
|
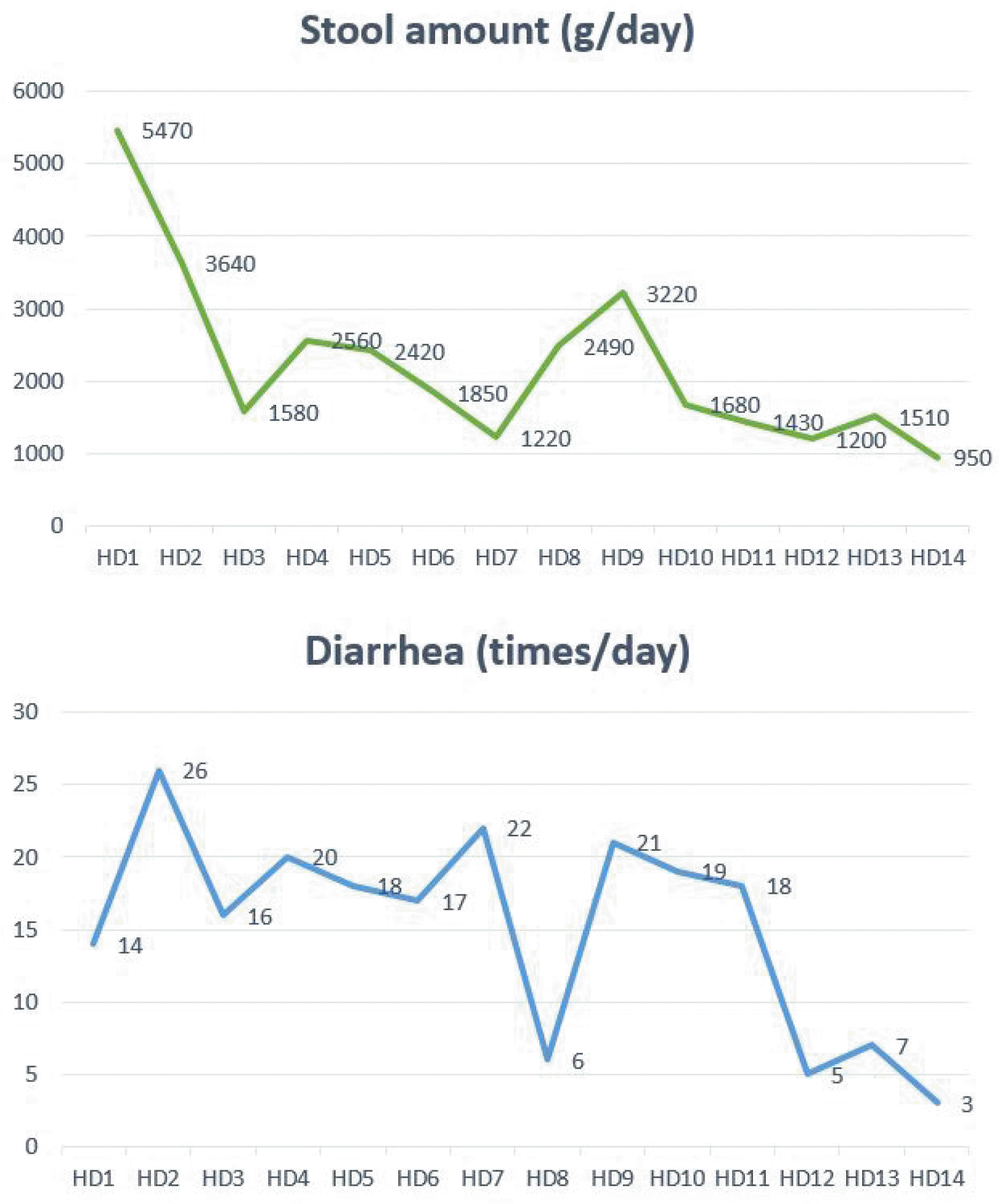 | Fig. 3Hospital course of the patient’s stool amount and the number of diarrhea episodes per day. 
|
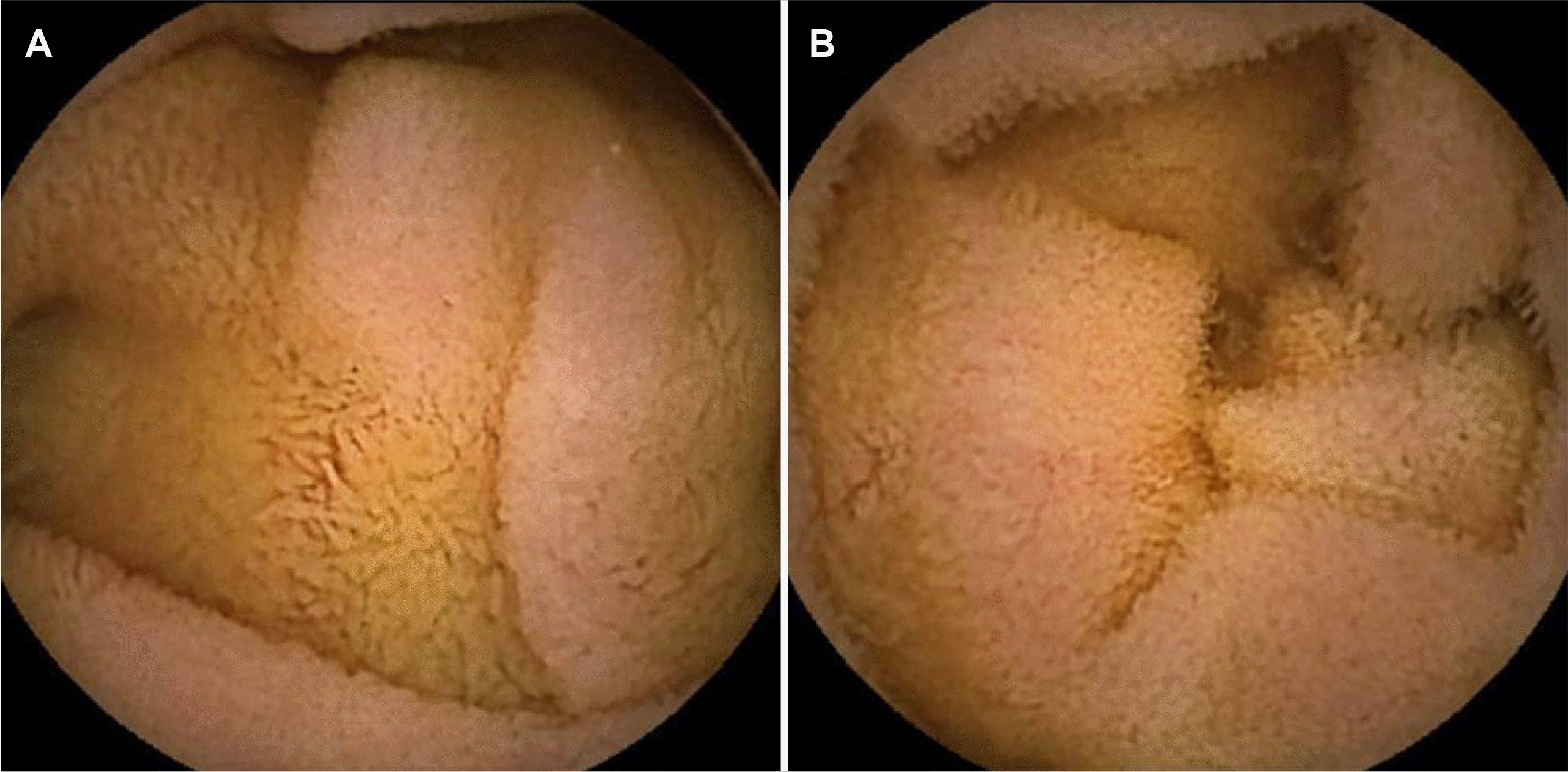 | Fig. 4Images of video capsule endoscopy showing recovered villi and the disappearance of edema on the (A) distal duodenum and (B) upper jejunum. 
|
Go to :

DISCUSSION
A few years after the introduction of olmesartan, a randomized study showed that there were no significant gastrointestinal side effects of the drug, compared with placebo.
3 However, cases of sprue-like OAE have been reported subsequently. In 2012, Rubio-Tapia et al.
2 identified 22 patients on olmesartan who developed the clinical features of chronic diarrhea, weight loss, and sprue-like enteropathy, evidenced by the atrophy of villi and mucosal inflammation on intestinal biopsy. All the cases demonstrated negative serology for coeliac disease by tTG antibody IgA, and a majority had a history of treatment failure with a gluten-free diet. Notably, all 22 patients had their symptoms resolved upon withdrawal of olmesartan and discontinuation of the gluten-free diet.
2 There are no reported cases of OAE in Korea, but 31 patients with suspected enteropathy were reported among the 108,559 patients who were initiated on angiotensin-converting enzyme inhibitors or ARBs in a Korean nationwide observational cohort study which used data from the Korean National Health Insurance Service.
6
Although the mechanism of olmesartan-induced enteropathy is unknown, it is assumed that it inhibits the transforming growth factor B and activates the angiotensin II type 2 receptor (AT
2R) by selectively blocking angiotensin II receptor type 1 receptors (AT
1R), thus inducing apoptosis of enterocytes.
7 This may ultimately lead to villous atrophy without an inflammatory reaction and an increase in intraepithelial lymphocytes.
7 The time taken for the development of these processes indicates the time interval between drug exposure and the onset of symptoms. A systematic review of 54 cases of olmesartan-induced sprue-like enteropathy showed a positivity rate of 64% for the human leukocyte antigen (HLA) DQ2/DQ8, whereas only 40% of the general population are positive for the same antigen, indicating the involvement of the HLA system in the disease as possible pathophysiology.
1
The symptoms and signs of OAE include diarrhea, nausea, vomiting, weight loss, and low albumin.
7 Our patient presented with watery diarrhea lasting over 8 weeks, and it was so severe that the subsequent AKI required CRRT. Although some severe AKI cases of sprue-like OAE have been reported by Roca-Argente et al.,
8 none of them were treated with dialysis therapy. Since the time interval between drug exposure and the onset of symptoms can vary widely from weeks to years,
5,7 it is essential to consider OAE as a possible diagnosis in patients presenting with such manifestations. The diagnosis of OAE can be made when villous atrophy and the inflammation of the small intestine are observed during duodenoscopy or VCE, coeliac serology is negative, and some other conditions including autoimmune, drug-induced, malignancy-associated, post-infectious enteropathies are excluded.
7 An association of OAE with some autoimmune diseases such as systemic sclerosis has been suggested in a few reports.
9-11 For symptom relief, oral loperamide has been shown to be effective as in this case, and budesonide or intravenous steroids can be used in cases with severe symptoms such as dehydration and/or suboptimal response to the cessation of olmesartan.
12 Although the disease course and severity spectrum of OAE are not clearly known, nearly all reported cases of OAE showed complete clinical improvement after the cessation of olmesartan.
1,2,5 Also, histologic recovery was documented in most patients during a mean follow-up interval of 242.3 days (range 54-707 days).
2
Enteropathies have also been reported with other ARBs. In a systematic review of previously reported ARB-associated enteropathy cases, causative medications were olmesartan (94.0%), telmisartan (2.0%), irbesartan (1.6%), valsartan (1.2%), losartan (0.8%), and eprosartan (0.4%).
5 The reason for the varying incidences among different drugs, within the same class, may be related to the differences in affinity to AT
1R versus AT
2R and the amount of drug administered. In addition, other drugs known to cause similar sprue-like enteropathy include mycophenolate mofetil, methotrexate, azathioprine, colchicine, and non-steroidal anti-inflammatory drugs.
13 Thus, a thorough investigation of possible causative drugs is essential before studies are undertaken to identify other causes of enteropathy.
Whether re-administration of olmesartan induces the recurrence of diarrheal symptoms is not clearly known. However, Dias et al.
14 reported some cases in which the reintroduction of olmesartan caused OAE again. In the case of our patient, we avoided re-administration of olmesartan since diarrhea was so profuse that it could have resulted in death. Replacing the antihypertensive medication with a drug of another class can be a reasonable choice.
14
Once diagnosed, it is not very challenging to treat OAE since the mainstay of treatment is the discontinuation of olmesartan with best supportive care. However, diagnosis of OAE is sometimes difficult as the manifestation can be heterogeneous with a probable involvement of any part of the gastrointestinal tract.
14 Also, there is no sex predilection, and the majority of OAE patients are reported to be in their seventh or eighth decades of life.
1 The case presented in this paper may be helpful in the differential diagnosis of patients with unexplained chronic diarrhea, enable diagnosis of OAE through the exclusion of other rare causes of chronic diarrhea for appropriate management of the condition.
Go to :


