Abstract
Background/Aims
The objective of this study was to determine the more appropriate wound-closure method by comparing the effectiveness of two methods in a group of patients who underwent ileostomy repair.
Methods
The study conducted after obtaining the approval of the Institutional Review Board (IRB) included 58 patients ≥19 years of age who underwent ileostomy at the Department of Surgery at the Presbyterian Medical Center. This was a retrospective, single-center trial. Patients who underwent ileostomy closure between January 2011 and September 2017 were assigned to the primary wound-closure (PC, n=25) group and the purse-string wound-closure (PSC, n=33) group. Post-repair complications, such as wound infection, delayed healing, and patient satisfaction related to wound management, were investigated and compared according to the wound-closure method.
Results
The PSC group had a significantly lower surgical site infection rate than the PC group (0% vs. 44%, p<0.001). The wound-healing period was also significantly different between the PC and PSC groups (mean 27.18 days vs. 20.96 days, p=0.023). However, the postoperative wound-healing delay of >30 days was not significantly different (39% vs. 20%, p=0.114). In addition, there were no significant differences in the response to questionnaires on patient satisfaction between the two groups.
Conclusions
PSC has a lower surgical site infection rate and the wound-healing delay was not very different from that of PC. Therefore, if patients are at risk of wound infection, such as in severe wound contamination, long operating time, and immunocompromised conditions, we should consider PSC as a wound closure method of choice.
Diverting ileostomy for anastomotic protection during ileoanal, coloanal, or colorectal anastomosis has become a popular technique.1-3 Ileostomy formation and repair are relatively easy and safe procedures that can effectively divert stools, with complication rates of <5%.4 With advances in surgical techniques, the introduction of preoperative chemoradiation therapy for low rectal cancer, and an increase in sphincter- sparing surgery, the frequency of diverting ileostomy has increased. Diverting ileostomy is also performed after proctocolectomy and ileal pouch-anal anastomosis in cases involving ulcerative colitis. Unlike other parts of the colon, the rectum has a high risk of anastomotic leakage, which warrants such a diverting procedure. This is because low anterior resection or coloanal anastomosis exerts high tension on the anastomotic site due to the loss of the ability to hold stools and the rectoanal inhibitory reflex, which are unique functions of the rectum when preoperative chemoradiation therapy is performed to treat rectal cancer.2 Moreover, ileostomies are also being performed for therapeutic purposes in cases of Crohn’s disease with perianal involvement or rectovaginal fistula. Therefore, ileostomies and subsequent ileostomy repairs are necessary procedures in these clinical scenarios.
During an ileostomy repair, a wound is created on the skin, including the subcutaneous layer, where the stoma is formed. This is a contaminated wound, and a relatively large dead space is created. Consequently, wound infection commonly occurs after skin closure, and wound healing may be delayed.5-11 Complications associated with postoperative wounds cause a decline in the quality of life and increased medical cost, length of hospital stay, and the number of hospital visits.12 Therefore, the objective of the present study was to determine the best wound-closure method by comparing the incidence rate of complications according to the wound-closure method in a group of patients who underwent ileostomy repair after multiple rectal cancer surgeries and some cases of stoma formation with trauma, inflammatory bowel, and acute diverticulitis.
After exempted from the Institutional Review Board (IRB), this retrospective study was initiated and included 58 patients ≥19 years of age who underwent ileostomy at the Department of Surgery at the Presbyterian Medical Center between January 1, 2011, and September 30, 2017. Medical records covering an average of 40 months after ileostomy were retrospectively analyzed. Moreover, post-repair complications, such as wound infections and delayed healing, and patient satisfaction related to wound management, were investigated and compared according to the wound-closure method.
The selection was randomized, but there were more patients in the primary wound-closure (PC) group at the beginning of the recruitment period, and more patients in the purse-string wound closure (PSC) group at the end of the recruitment period. This was because, from 2011 to 2014, PC was more commonly performed first, but when the frequency of infections increased, PSC became more common after 2014.
The patients were divided into PC and PSC groups according to the wound-closure method used. Among the 66 patients who were included in the study, 25 were assigned to the PC group and 33 were assigned to the PSC group, after excluding eight patients with delayed PC (Fig. 1). Age, sex, cause of surgery, stoma type (loop type and end type), diabetes mellitus status, pre-repair chemotherapy, wound-drainage tube insertion, BMI, American Society of Anesthesiologists score, and the smoking status of patients in each group were investigated. For perioperative variables, the duration between ileostomy formation and repair (repair interval), operative time, mean hospital stay, and laboratory test results were investigated. Comparisons were made between the groups, and the statistical significance was analyzed.
For the skin incision, a circular incision was made 2 mm lateral to the stoma mucocutaneous margin, after which the cylindrical stoma was separated from the abdominal wall. For small intestinal anastomosis, lateral-lateral anastomosis was performed by automatic stapling, while the fascia layer was approximated by a continuous suture using looped 0/0 polydioxanone.
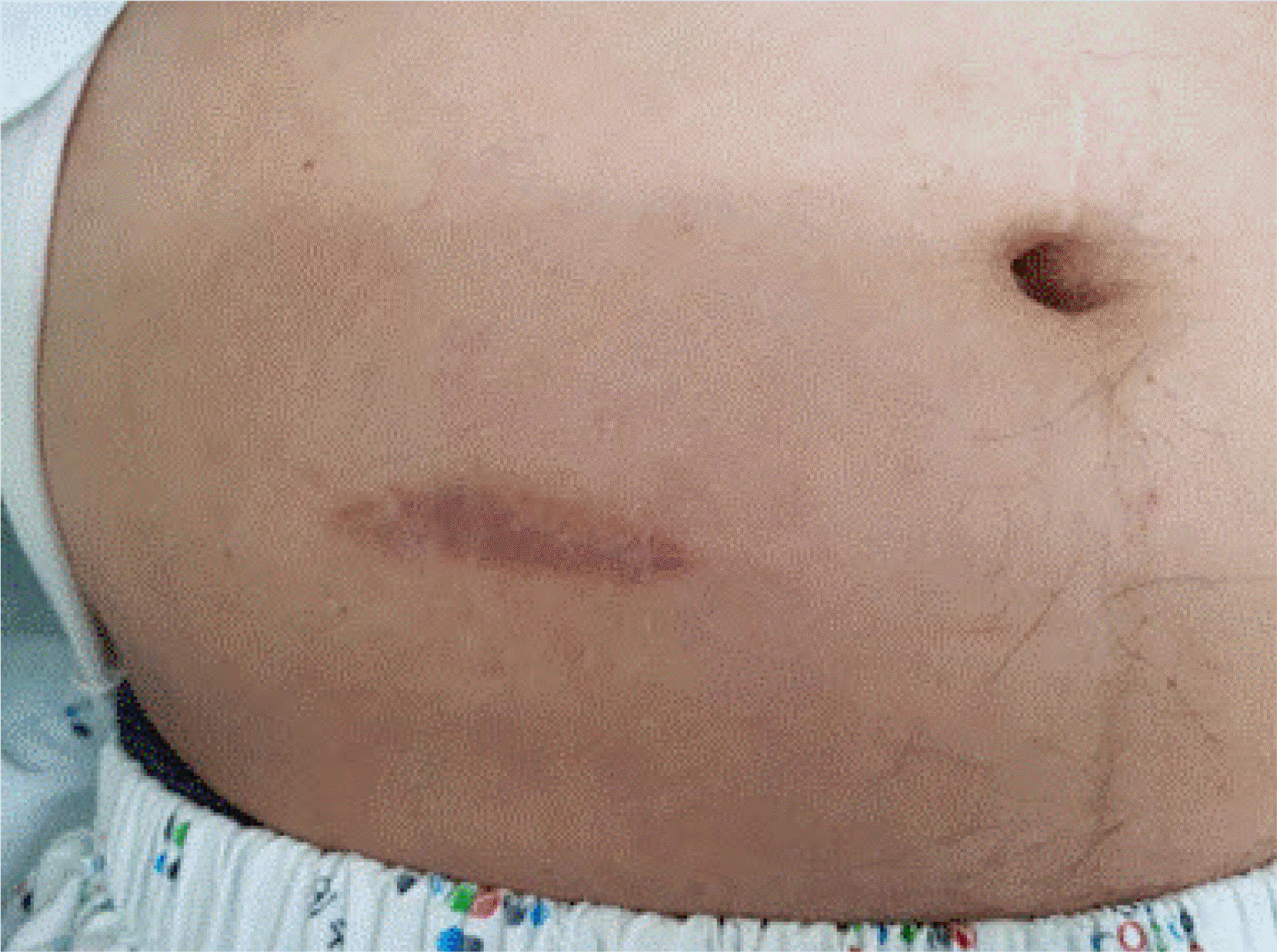
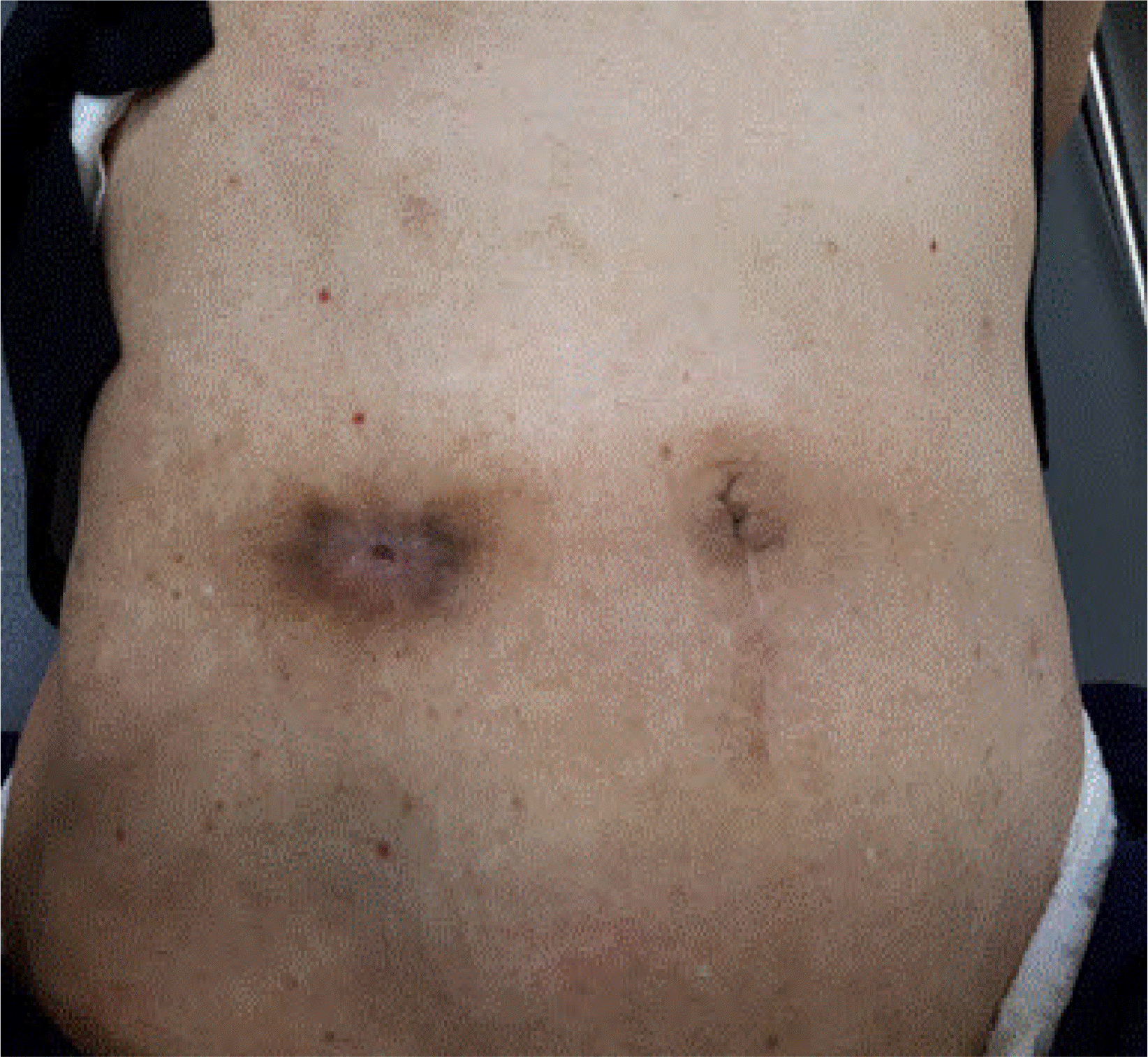
Subsequently, the subcutaneous layer was approximated using 2/0 vicryl sutures in the PC group, followed by skin approximation by interrupted suturing with four or five stitches using 2 or 3/0 nylon. In the PSC group, the subcutaneous layer was left unapproximated or only one or two sutures were used, after which the dermal layer was approximated by continuous suturing using 0/0 nylon, and a drainage window (approximately 7-10 mm) was retained (Fig. 2).
All the patients in both groups were administered prophylactic antibiotics (second-generation cephalosporin) prior to the skin incision. Moreover, the anastomotic site and other color lesions were checked by sigmoidoscopy or colonoscopy prior to the stoma reversal in all patients. All patients were operated upon under general anesthesia and were allowed to start on a fluid diet after flatulence.
The primary outcomes measured were the incidence of postoperative wound infections, the incidence of delayed wound healing, and patient satisfaction with respect to wound management. Wound infection was defined as the presence of purulent exudate in the wound, tenderness, redness, induration, and/or fever, and positive bacterial culture results. During hospitalization, the wound from the ileostomy repair was managed by the surgical staff with daily monitoring. The patients in the PSC group were trained to manage their wound at home after discharge, to clean the skin around the drainage window and the wound with a sterile cotton swab or pad while wearing sanitary gloves, and to insert Aquacel Ag® (ConvaTec Inc., Greensboro, NC, USA) which has an antibacterial effect into the drainage window and cover the affected area with gauze, until the drainage window decreased to a certain size. The patients visited the outpatient department 7 days and one month after discharge. Patients who required additional wound management were treated during additional outpatient visits. Delayed wound healing was defined as an open wound 30 days after surgery. All surgery-related outcomes were determined through a review of electronic medical records.
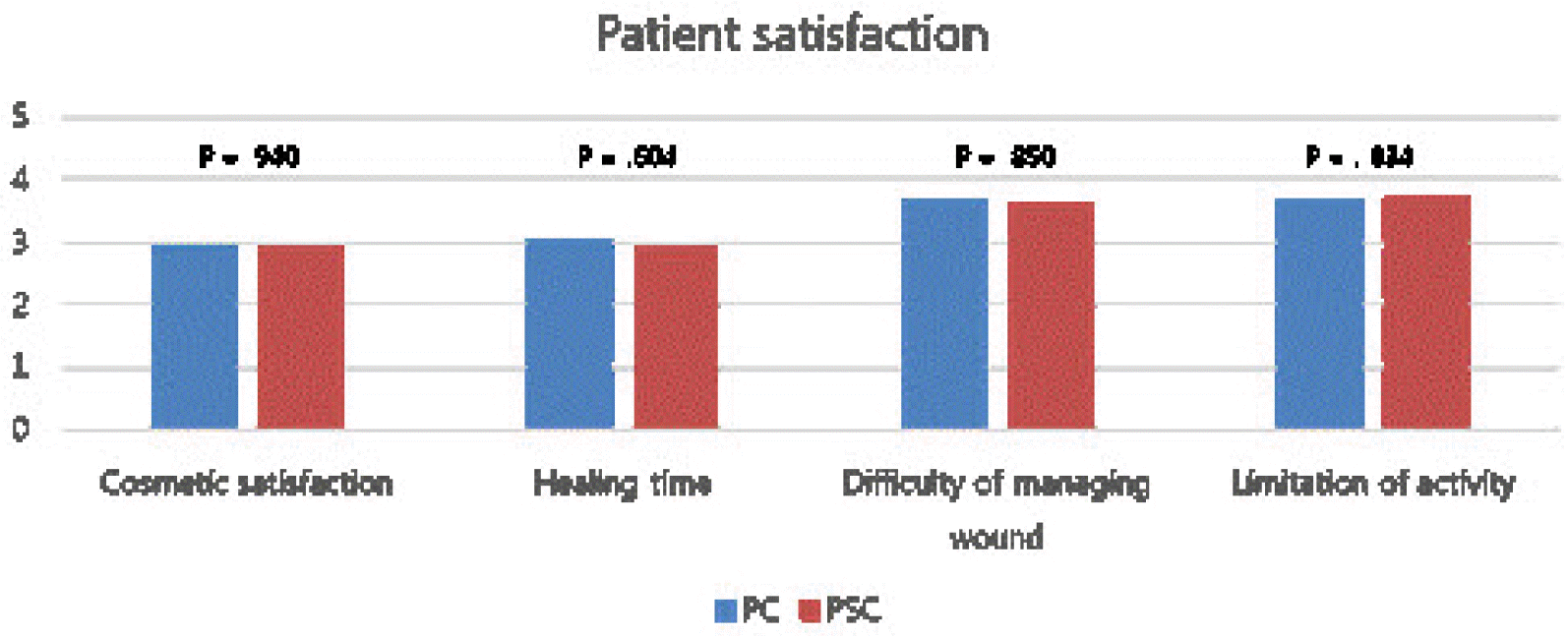
Cosmetic satisfaction, satisfaction with the wound healing time, satisfaction with the difficulty in managing the wound, and limitations in activities were investigated via telephonic conversations using a 5-point Likert scale. Cosmetic satisfaction was measured on a scale of “very dissatisfied” (1 point), “somewhat dissatisfied” (2 points), “average” (3 points), “somewhat satisfied” (4 points), and “very satisfied” (5 points) (Figs. 3, 4). The satisfaction with wound healing time was measured on a scale of “felt very long” (1 point), “felt somewhat long” (2 points), “adequate” (3 points), “felt somewhat short” (4 points), and “felt very short” (5 points). The difficulty in managing the wound was measured on a scale of “very difficult” (1 point), “somewhat difficult” (2 points), “average” (3 points), “somewhat easy” (4 points), and “very easy” (5 points). The limitations in activities were measured on a scale of “limited” (1 point), “somewhat limited” (2 points), “average” (3 points), “almost no limitation” (4 points), and “no limitation” (5 points).
Statistical analyses were performed using the SPSS 24.0 (IBM Cooperation, New York, NY, USA) statistics program. Categorical data were analyzed using the chi-square test, while the continuous data were analyzed by mean comparison using the Student’s t-test. The risk factors of wound infection were analyzed using the logistic regression test. Results with a p-value ≤0.05 were considered statistically significant.
The study population included 58 patients with a mean age of 64.1 years. Of these, 25 (43%) were assigned to the PC group and 33 (57%) to the PSC group. There were 42 men (72%) and 16 women (28%), and the mean repair interval was 148 days (21 weeks). With respect to the underlying disease necessitating surgery included 51 cases of malignancy (88%), two cases of inflammatory bowel disease (3%), and five cases of other diseases, including trauma and infectious diseases (9%). With respect to stoma type, there were 52 cases of loop-type (90%) and 6 cases of end-type (10%) stoma. All the stoma-reversal surgeries were performed as planned.
The results showed no statistically significant differences in the baseline characteristics between the two groups, except age and wound-drainage tube insertion (Table 1).
The results also showed no statistically significant differences in the perioperative variables between the two groups, except in serum creatinine levels taken just before stoma restoration (usually 2-7 days before surgery) (Table 2).
The ileostomy reversal wound infection rate showed a significant difference between the PC and PSC groups in the chi-square test (44% vs. 0%, respectively, p<0.01) (Table 3). To treat patients with wound infection, the sutures or staples used for skin closure were removed, after which the wound was opened and disinfected. The wound was allowed to heal by secondary intention, and when necessary, the area was disinfected for a certain period, and re-approximated. In the chi-square test on the association between the wound-infection group and other factors, the results showed a significant association with surgery type (p<0.001) and diabetes mellitus (p=0.034) (Table 4). Univariate logistic regression analysis showed that diabetes mellitus was a risk factor associated with wound infection (p=0.046), but multivariable logistic regression analysis showed no association between the factors and wound infection (Table 5).
The results showed a significant difference in mean wound-healing time between the PC and PSC groups (20.96 vs. 27.18 days, respectively, p=0.023). However, there was no significant difference in the rate of delayed wound healing over a 30-day period between the PC and PSC groups (20% vs. 39%, respectively, p=0.114) (Table 3).
Moreover, there was a significant difference in mean wound-healing time between the PC groups with and without wound infection (27.00 vs. 16.21 days, respectively, p=0.004).
A patient satisfaction survey was conducted to evaluate cosmetic satisfaction, satisfaction with the wound healing time, satisfaction with the difficulty in managing the wound, and the limitations in activities. The results indicated that none of the items showed significant differences between the two groups (Table 3, Fig. 5).
There are few options for the skin-closure technique used during ileostomy repair. The most widely known method is PC, and an alternative method is PSC, which typically has a lower wound-infection rate.13-19
In general, wound-healing methods can be divided into primary, secondary, and tertiary intentions.12 Primary intention is a method that uses sutures for immediate wound approximation when there is no wound contamination and less tissue deficit. In the present study, PC corresponded to primary intention. PC enables wound healing within a relatively short period, but because stoma reversal involves operating on an open intestine, the wound from the ileostomy repair could be considered a contaminated wound, which has a higher likelihood of wound infection than a clean wound. A secondary intention is a method used for a contaminated wound or in cases with a relatively large tissue deficit. This method promotes wound healing through the proliferation of granulation tissue by disinfecting the wound in an open state without approximation. In the present study, this corresponds to PSC. Since an ileostomy wound is a contaminated wound and a relatively large dead space could be created, it is considered that PSC may be a more appropriate method for ileostomy-repair wounds than PC. The tertiary intention is delayed primary closure, and the present study did not include a patient group for this method. This method can reduce wound infection and shorten delays in wound healing, which is a known disadvantage of the secondary intention. However, a retrospective study by Harold et al.20 and a randomized study by Lahat et al.21 reported that a comparison of the rate of ileostomy- repair wound infections between PC and delayed wound-closure groups showed a significantly higher rate of wound infections in the delayed wound-closure group than in the PC group.
In the present study, the PC group showed a wound infection rate of 44%, which was higher than the known rate of 18.0-36.6%.6,7 The present study used 2/0 vicryl for subcutaneous approximation in PC, but this suture may have acted as a foreign body to increase the wound-infection rate.12 Moreover, reports indicate that approximately 25% of the vicryl sutures remain unabsorbed even after 4 weeks, and because the sutures are braided, their use is considered inappropriate for contaminated wounds.22 There have been various attempts to reduce the wound-infection rate, including the injection of antibiotics into the wound and insertion of a drainage tube.23 The present study also investigated the association between drainage tube-insertion and wound-infection but this was not significant (p=0.38).
The dermal layer was tightened after PSC, which is known to be advantageous in preventing wound infections because the wound is disinfected continuously by leaving a drainage tube and not closing the skin completely.17,24 The results of the present study also showed a wound infection rate of 0% in the PSC group, which was significantly lower than that of the PC group (p<0.01). Based on these results, it could be inferred that the use of PSC is more advantageous than PC in terms of wound-infection rates.
However, the disadvantage of PSC is the long time to complete wound healing depending on the patient and this was identified in studies by other authors.6,7,14 In the present study, the mean wound-healing time in the PC and PSC groups was 20.96 and 27.18 days, respectively (p=0.023), while the percentage of cases with delayed wound healing of ≥30 days in the PC and PSC groups was 20% and 39%, respectively, although the difference was not statistically significant (p=0.114).
In the comparison between the 11 cases of wound infection in the PC and PSC groups, there was no statistically significant difference in the mean wound-healing time (27.00 vs. 27.18 days, respectively; p=0.960) and delayed wound healing of ≥30 days (36% vs. 39%, respectively, p=0.858). Based on these results, it was determined that although wound infection occurred in the PC group, there was no significant difference in the healing time as compared to the PSC group. However, wound infection causes a decline in the quality of life and increased medical cost, length of hospital stay, and the number of hospital visits, while also increasing the risk of complications such as incisional hernia. Therefore, efforts should be made to avoid wound infection.12,25
In this study, the role of the patient in wound management and the education about the patient were important. Currently, the number of patients requiring continuous management is increasing due to the rise in the prevalence of chronic diseases, with proportionate increases in medical costs.26 Hence, the concept of therapeutic patient education (TPE) was introduced, and TPE has been developed for asthma, pulmonary insufficiency, cancer, and inflammatory bowel disease and, in particular, for patients with stoma or wounds.
According to the World Health Organization, TPE is education provided by healthcare providers and is designed to enable a patient (or a group of patients and families) to manage the treatment of their condition and prevent avoidable complications while maintaining or improving the quality of life. Its principal purpose is to support all other interventions including pharmacologic, physical therapy, and so on with patient understanding and adherence. Patient education on wound management was specifically introduced in the management of diabetic wounds in the 1970s, and an intensive care center and team were set up subsequently, leading to more professional education and management. This has resulted in reduced personal and community health care costs.27 In the future, it is expected that more effective education and management will be possible through remote treatment or social media video clips.
Previous studies have reported high cosmetic satisfaction as an advantage of PSC.5,7,24 However, there was no significant difference between the two groups with respect to cosmetic satisfaction, satisfaction with the wound healing time, satisfaction with the difficulty in managing the wound, and the limitations in activities. The patients considered the treatment of the underlying disease and stoma reversal to be more important than the cosmetic aspects, while some responded that the wound was not conspicuous because it was located on the lower right part of the abdomen.
A simple technique has been designed to reduce postoperative dead space after ileostomy repair with improved cosmetic outcomes.28 This technique, which focuses more on ileostomy formation than ileostomy repair, uses a transverse skin incision (approximately 3-4 cm long) and no incision of subcutaneous tissue during ileostomy formation, instead of the circular skin incision and cylindrical subcutaneous dissection. This ensures that no dead space is created during repair, which could reduce infection and promote faster wound healing. Therefore, a more favorable cosmetic outcome can be achieved by minimizing the wound through PC.
The chi-square test on factors associated with wound infection identified the “operation type” and “diabetes mellitus,” while a specific association with preoperative chemotherapy, BMI, operative time, and preoperative laboratory test results could not be found. However, multivariate regression analysis with controlled variables showed that none of the factors were significantly associated with wound infections. According to a randomized study by Lee et al.,6 the incidence of wound infection was associated with delayed wound healing. In the present study, however, the statistical analysis of results from all patient groups produced no significant results, while a significant association between the incidence of wound infection and delayed wound healing was found between the wound infection and non-infection subgroups within the PC group (27.00 vs. 16.21 days, respectively, p<0.004).
With respect to the time point for repair, a study by Perez et al.13 reported that repair after 8.5 weeks reduces the morbidity of the surgery. In the present study, the mean repair interval was 21 weeks, which was slightly longer than that reported in other studies. It is believed that these results may be due to a high percentage (88%) of patients with malignancy as the underlying disease, which resulted in these patients undergoing an ileostomy repair procedure after postoperative adjuvant chemotherapy. However, the present study, as well as other studies, have found that chemotherapy is not associated with wound infection or delayed wound healing; therefore, it is believed that performing the repair procedure after chemotherapy has no influence on wound-related adverse events.8
The present study has some limitations. First, it was a small-sized retrospective study. Second, because the questionnaire survey was conducted several years after the surgery, recall bias may have occurred. Third, there may also be statistical bias due to the inclusion of multiple surgeons.
PC is a relatively simple procedure, with rapid wound healing. While it showed a higher rate of wound infection, the healing time was shorter than that of PSC. Therefore, considering PC as the first wound closure method would be a valid option while applying a strict procedure for minimizing infection during ileostomy formation and repair. PSC had a much lower wound infection rate and showed no major differences in delayed wound healing ≥30 days compared to PC. Therefore, PSC may be considered in cases with factors associated with a high risk of wound infection, such as severe wound contamination, long operative time, or immunosuppressed patients.12 In other words, it is important to select the technique suitable for the given situation by recognizing the advantages and disadvantages of each method, rather than considering only one method. Further, more objective results may be obtained through prospective randomized controlled studies in the future.
REFERENCES
1. Song GW, Yu CS, Lee HO, et al. 2003; Ileostomy related complications. J Korean Soc Coloproctol. 19:82–89. DOI: 10.1245/s10434-013-3287-9,. PMID: 24085329. PMCID: PMC4026258.
2. Kim HY, Kim IY, Kim SH, Yoon KS. 1997; Evaluation of the usefulness of loop ileostomy during low anterior resection or. J Korean Soc Coloproctol. 13:397–402.
3. Cipe G, Erkek B, Kuzu A, Gecim E. 2012; Morbidity and mortality after the closure of a protective loop ileostomy: analysis of possible predictors. Hepatogastroenterology. 59:2168–2172. DOI: 10.5754/hge12115. PMID: 22440245.

4. Phang PT, Hain JM, Perez-Ramirez JJ, Madoff RD, Gemlo BT. 1999; Techniques and complications of ileostomy takedown. Am J Surg. 177:463–466. DOI: 10.1016/S0002-9610(99)00091-4. PMID: 10414694.

5. Hsieh MC, Kuo LT, Chi CC, Huang WS, Chin CC. 2015; Pursestring closure versus conventional primary closure following stoma reversal to reduce surgical site infection rate: a meta-analysis of randomized controlled trials. Dis Colon Rectum. 58:808–815. DOI: 10.1097/DCR.0000000000000401. PMID: 26163961.

6. Lee JT, Marquez TT, Clerc D, et al. 2014; Pursestring closure of the stoma site leads to fewer wound infections: results from a multicenter randomized controlled trial. Dis Colon Rectum. 57:1282–1289. DOI: 10.1097/DCR.0000000000000209. PMID: 25285695.
7. Camacho-Mauries D, Rodriguez-Díaz JL, Salgado-Nesme N, González QH, Vergara-Fernández O. 2013; Randomized clinical trial of intestinal ostomy takedown comparing pursestring wound closure vs conventional closure to eliminate the risk of wound infection. Dis Colon Rectum. 56:205–211. DOI: 10.1097/DCR.0b013e31827888f6. PMID: 23303149.

8. Lee DH, Yun JA, Jung KU, et al. 2012; Comparison of morbidity between preventive and therapeutic ileostomy repair. Korean J Clin Oncol. 8:44–49. DOI: 10.14216/kjco.12006.

9. Williams LA, Sagar PM, Finan PJ, Burke D. 2008; The outcome of loop ileostomy closure: a prospective study. Colorectal Dis. 10:460–464. DOI: 10.1111/j.1463-1318.2007.01385.x. PMID: 17953706.

10. El-Hussuna A, Lauritsen M, Bülow S. 2012; Relatively high incidence of complications after loop ileostomy reversal. Dan Med J. 59:A4517. PMID: 23158893.
11. Chow A, Tilney HS, Paraskeva P, Jeyarajah S, Zacharakis E, Purkayastha S. 2009; The morbidity surrounding reversal of defunctioning ileostomies: a systematic review of 48 studies including 6,107 cases. Int J Colorectal Dis. 24:711–723. DOI: 10.1007/s00384-009-0660-z. PMID: 19221766.

12. Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. 1999; Guideline for prevention of surgical site infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 27:97–96. DOI: 10.1016/S0196-6553(99)70088-X. PMID: 10196487.
13. Perez RO, Habr-Gama A, Seid VE, et al. 2006; Loop ileostomy morbidity: timing of closure matters. Dis Colon Rectum. 49:1539–1545. DOI: 10.1007/s10350-006-0645-8. PMID: 16897328.

14. Banerjee A. 1997; Pursestring skin closure after stoma reversal. Dis Colon Rectum. 40:993–994. DOI: 10.1007/BF02051210. PMID: 9269819.

15. Li LT, Hicks SC, Davila JA, et al. 2014; Circular closure is associated with the lowest rate of surgical site infection following stoma reversal: a systematic review and multiple treatment meta-analysis. Colorectal Dis. 16:406–416. DOI: 10.1111/codi.12556. PMID: 24422861.

16. Reid K, Pockney P, Pollitt T, Draganic B, Smith SR. 2010; Randomized clinical trial of short-term outcomes following purse-string versus conventional closure of ileostomy wounds. Br J Surg. 97:1511–1517. DOI: 10.1002/bjs.7151. PMID: 20575111.

17. Sutton CD, Williams N, Marshall LJ, Lloyd G, Thomas WM. 2002; A technique for wound closure that minimizes sepsis after stoma closure. ANZ J Surg. 72:766–767. DOI: 10.1046/j.1445-2197.2002.02514.x. PMID: 12534395.

18. McCartan DP, Burke JP, Walsh SR, Coffey JC. 2013; Purse-string approximation is superior to primary skin closure following stoma reversal: a systematic review and meta-analysis. Tech Coloproctol. 17:345–351. DOI: 10.1007/s10151-012-0970-y. PMID: 23354904.

19. Mirbagheri N, Dark J, Skinner S. 2013; Factors predicting stomal wound closure infection rates. Tech Coloproctol. 17:215–220. DOI: 10.1007/s10151-012-0908-4. PMID: 23076288.

20. Harold DM, Johnson EK, Rizzo JA, Steele SR. 2010; Primary closure of stoma site wounds after ostomy takedown. Am J Surg. 199:621–624. DOI: 10.1016/j.amjsurg.2010.01.008. PMID: 20466105.

21. Lahat G, Tulchinsky H, Goldman G, Klauzner JM, Rabau M. 2005; Wound infection after ileostomy closure: a prospective randomized study comparing primary vs. delayed primary closure techniques. Tech Coloproctol. 9:206–208. DOI: 10.1007/s10151-005-0228-z. PMID: 16328128.

22. Townsend CM, Beauchamp RD, Evers BM. 2016. Sabiston textbook of surgery. 20th ed. Elsevier;Amsterdam:
23. Haase O, Raue W, Böhm B, Neuss H, Scharfenberg M, Schwenk W. 2005; Subcutaneous gentamycin implant to reduce wound infections after loop-ileostomy closure: a randomized, double-blind, placebo-controlled trial. Dis Colon Rectum. 48:2025–2031. DOI: 10.1007/s10350-005-0164-z. PMID: 16228839.

24. Milanchi S, Nasseri Y, Kidner T, Fleshner P. 2009; Wound infection after ileostomy closure can be eliminated by circumferential subcuticular wound approximation. Dis Colon Rectum. 52:469–474. DOI: 10.1007/DCR.0b013e31819acc90. PMID: 19333048.

25. Murray BW, Cipher DJ, Pham T, Anthony T. 2011; The impact of surgical site infection on the development of incisional hernia and small bowel obstruction in colorectal surgery. Am J Surg. 202:558–560. DOI: 10.1016/j.amjsurg.2011.06.014. PMID: 21924402.

26. Chapel JM, Ritchey MD, Zhang D, Wang G. 2017; Prevalence and medical costs of chronic diseases among adult medicaid beneficiaries. Am J Prev Med. 53:S143–S154. DOI: 10.1016/j.amepre.2017.07.019. PMID: 29153115. PMCID: PMC5798200.

27. Lataillade L, Chabal L. 2021; Therapeutic patient education: a multifaceted approach to ostomy care. Adv Skin Wound Care. 34:36–42. DOI: 10.1097/01.ASW.0000722756.35017.02. PMID: 33323801.

28. Keating J, Kelly EW, Hunt I. 2003; Save the skin and improve the scar: a simple technique to minimize the scar from a temporary stoma. Dis Colon Rectum. 46:1428–1429. DOI: 10.1007/s10350-004-6763-2. PMID: 14530688.
Fig. 2
Purse-string closure. The subcuticular continuous suture was performed using nylon 1/0 and then the wound was approximated. It has a 7-10-mm opening which facilitates wound drainage.
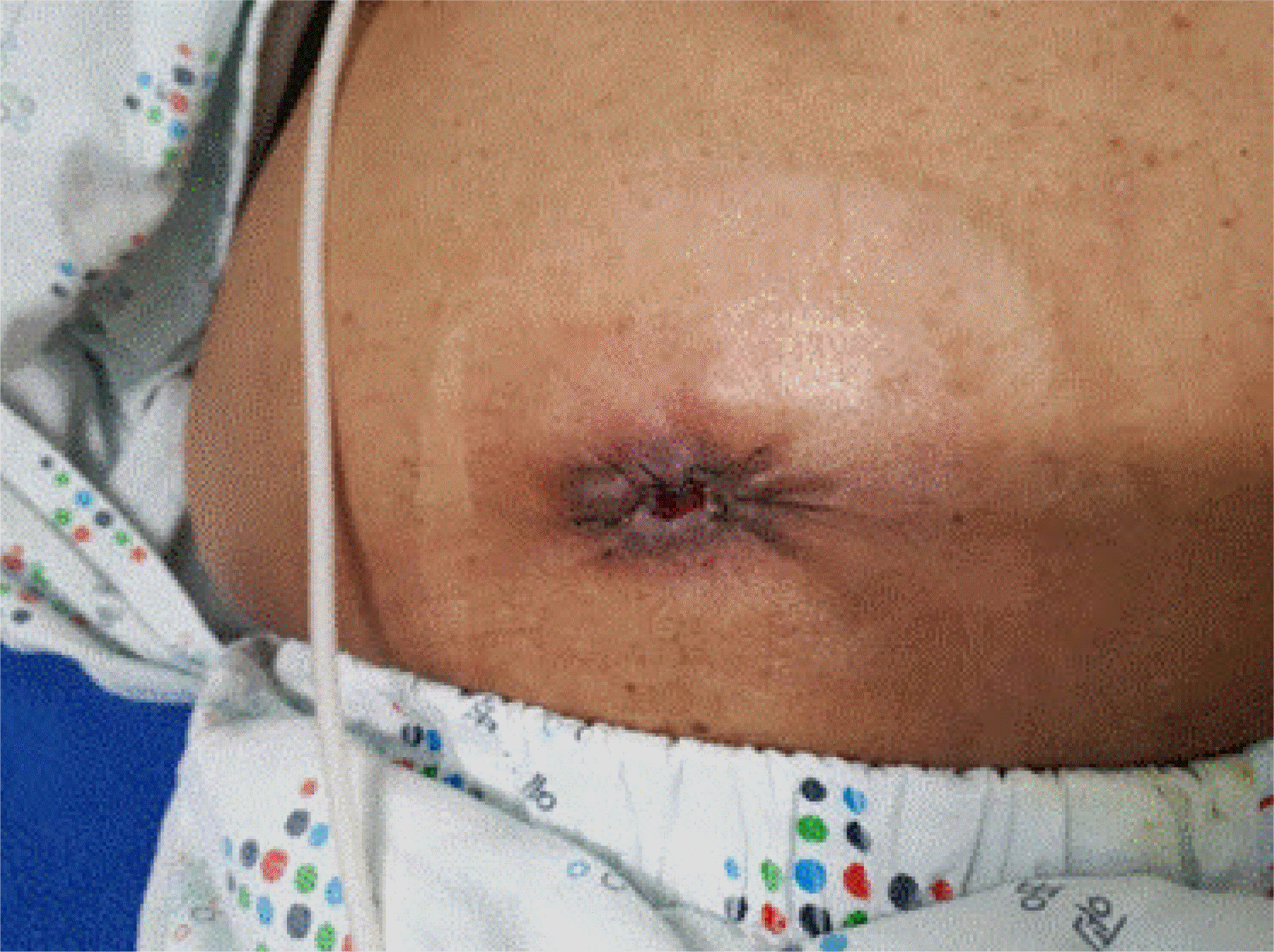
Table 1
Baseline Patient Characteristics
| Primary closure (n=25) | Purse-string closure (n=33) | Total (n=58) | p-value | |
|---|---|---|---|---|
| Age (years) | 60.80±10.38 | 67.24±8.86 | 0.014 | |
| Body Mass Index (kg/m2) | 23.21±3.56 | 21.70±5.08 | 0.209 | |
| Diabetes melitus | 6 (24.0) | 3 (9.0) | 9 | 0.120 |
| Cause of Surgery | 0.366 | |||
| Rectal cancer | 19 (76).0 | 29 (79.0) | 48 | |
| Right colon cancer | 1 (4.0) | 2 (6.0) | 3 | |
| Inflammatory bowel disease | 1 (4.0) | 1 (2.5) | 2 | |
| Etca | 4 (16.0) | 1 (2.5) | 5 | |
| Wound drainage | 0.015 | |||
| No | 19 (76.0) | 32 (97.0) | 51 | |
| Yes | 6 (24.0) | 1 (3.0) | 7 | |
| Pre-repair chemotherapy | 0.777 | |||
| No | 15 (60.0) | 22 (67.0) | 37 | |
| Yes | 10 (40.0) | 11 (33.0) | 21 | |
| Type of stoma | 0.719 | |||
| End type | 3 (12.0) | 3 (9.0) | 6 | |
| Loop type | 22 (88.0) | 33 (91.0) | 52 | |
| Smoking | 0.883 | |||
| No | 23 (92.0) | 30 (91.0) | 53 | |
| Yes | 2 (8.0) | 3 (9.0) | 5 | |
| ASA score | 0.121 | |||
| 1 | 6 (24.0) | 2 (6.1) | ||
| 2 | 15 (60.0) | 22 (66.7) | ||
| 3 | 4 (16.0) | 9 (27.3) |
Table 2
Perioperative Variables
Table 3
Comparison of Primary End-point Results
Table 4
Analysis of Factors Related to Stoma Site Infection
| Surgical site infection | Total (n=58) | p-value | ||
|---|---|---|---|---|
|
|
||||
| Positive (n=11) | Negative (n=47) | |||
| Cause of surgery | 0.296 | |||
| Rectal cancer | 7 (63.6) | 41 (87.2) | 48 | |
| Right colon cancer | 1 (9.1) | 2 (4.3) | 3 | |
| Inflammatory bowel disease | 1 (9.1) | 1 (2.1) | 2 | |
| Etca | 2 (18.2) | 3 (6.4) | 5 | |
| Wound drainage | 3 (27.3) | 4 (8.5) | 7 | 0.086 |
| Pre-repair chemotherapy | 5 (45.5) | 17 (36.2) | 22 | 0.568 |
| Type of operation | <0.001 | |||
| Primary closure | 11 (100.0) | 14 (29.8) | 25 | |
| Purse-string closure | 0 (0.0) | 33 (70.2) | 33 | |
| Diabetes melitus | 4 (36.4) | 5 (10.6) | 9 | 0.034 |
| Smoking | 0 (0.0) | 5 (10.6) | 5 | 0.258 |
| ASA score | 0.773 | |||
| 1 | 2 (19.0) | 6 (2.0) | 8 | |
| 2 | 6 (55.0) | 31 (66.0) | 37 | |
| 3 | 3 (27.0) | 10 (21.0) | 13 | |
| Operation time (min) | 109.2 | 108.1 | 0.558 | |
Table 5
Univariable and Multivariable Analyses of Risk Factors Associated with Stoma Site Infection
| Variable Univariable | p-valuea | Multivariable p-valuea |
|---|---|---|
| Age | 0.162 | 0.964 |
| Gender | 0.979 | |
| Men | 0.964 | |
| Women | - | |
| Cause of surgery | 0.347 | |
| Rectal cancer | 0.964 | |
| Right colon cancer | 0.989 | |
| Inflammatory bowel disease | 1.000 | |
| Etcb | - | |
| Type of operation | 0.998 | |
| Primary closure | 0.964 | |
| Purse-string closure | - | |
| Wound drainage | 0.103 | 0.964 |
| Pre-repair chemotherapy | 0.569 | 0.964 |
| Diabetes melitus | 0.046 | 0.965 |
| Smoking | 0.999 | 0.990 |
| ASA score | 0.775 | 0.999 |
| Operation time (min) | 0.558 | 0.963 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download