Abstract
Solid pseudopapillary pancreatic neoplasms are rare. The male-to-female ratio is 1:9, and metastasis occurs only in a few cases. A 39-year-old male with a solid pseudopapillary neoplasm (SPN) with lymph node metastasis underwent ultrasonography, CT, and MRI, which revealed a mass (8 cm) in the pancreatic head. Fluorodeoxyglucose (FDG)-PET showed a hypermetabolic lymph node in the root area of the superior mesenteric artery (SMA). The patient underwent pylorus-preserving pancreaticoduodenectomy, which confirmed a peripancreatic lymph node metastasis. The lymph node of the SMA root area remained because of the encasing of the superior mesenteric artery. After 14 months of follow-up (with no adjuvant therapy initiated), the residual metastatic lymph nodes showed no change and no recurrence. In conclusion, surgery of the primary tumor for patients with SPN is recommended, even in cases with metastatic lymph nodes remaining.
A solid pseudopapillary neoplasm (SPN) of the pancreas is a rare neoplasm, accounting for approximately 1-3% of pancreatic neoplasms. Most SPNs have a favorable prognosis because of their slow progression and low malignancy potential.1 They occur predominantly in young females. Less than 10% of patients with SPN are males.2 There are no significant differences in surgical treatment or prognosis between female and male patients.3 In addition, metastasis occurs in only 10-15% of patients. The 5-year survival rate is approximately 95-97%. This paper presents a rare case of SPN of the pancreas with lymph node metastasis in a male patient.
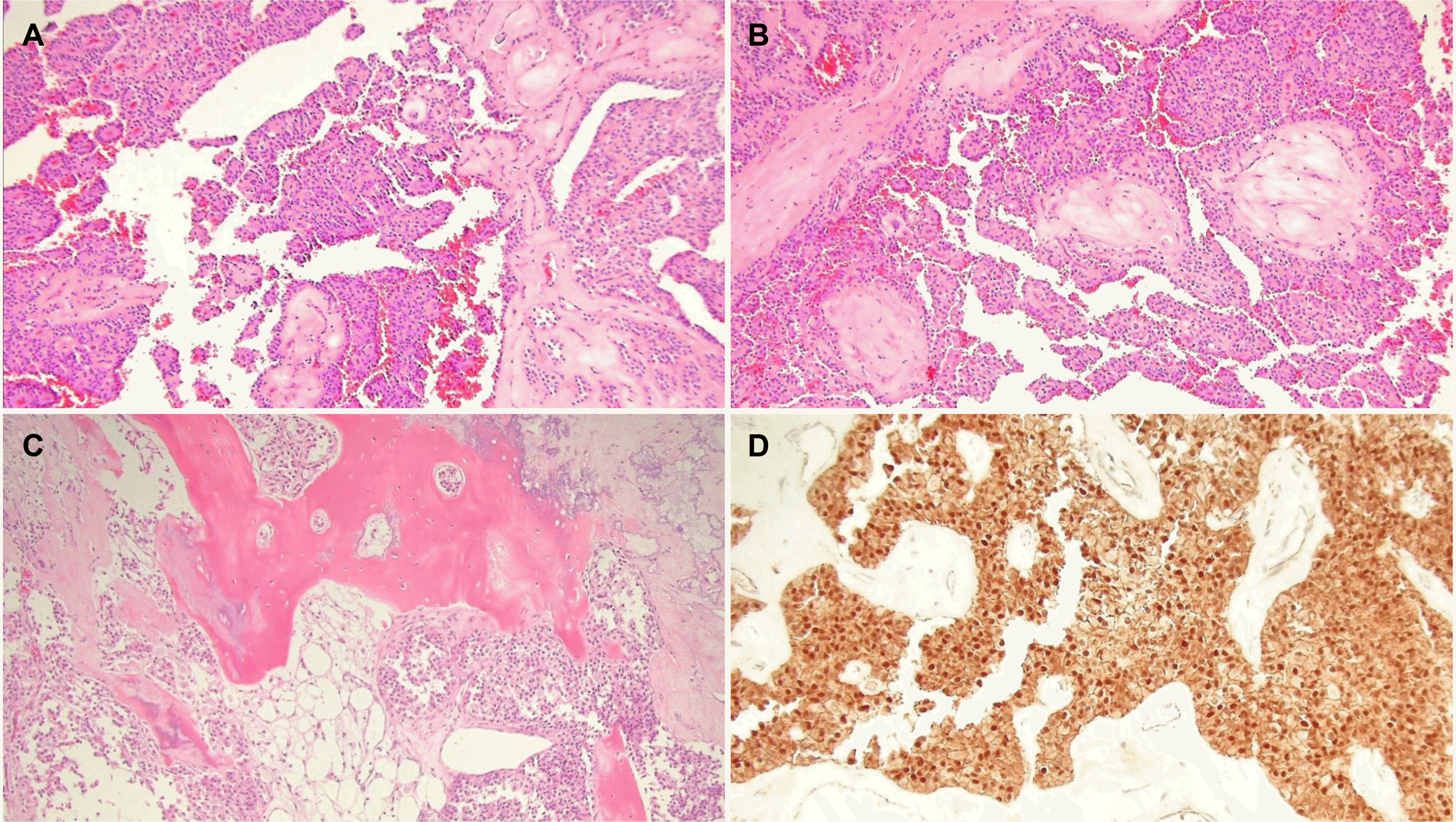
A 39-year-old male patient without underlying disease was referred to hospital owing to the incidental detection of a mass of the pancreatic head on a CT scan performed during a general health check-up. He was asymptomatic. On examination, the abdomen was soft with no palpable mass. The general examination was unremarkable. His vital signs were stable. The laboratory data, including AST 22 U/L (normal range 0-40 U/L), ALT 26 U/L (normal range 0-40 U/L), ALP 107 U/L (normal range 30-120 U/L), total bilirubin 1.0 mg/dL (normal range 0.2-1.2 mg/dL), amylase 46 U/L (normal range 22-80 U/L), and lipase 5 U/L (normal range 0-67 U/L) levels, were within the normal limits. The tumor markers, CA 19-9 5.02 U/mL (normal range 0-27 U/mL), CEA 1.05 ng/mL (normal range 0.0-5.0 ng/mL) were within the normal range. Abdominal ultrasonography (US) revealed a peripheral calcified soft tissue mass in the portocaval area (Fig. 1). CT of the pancreas revealed a mixed solid and cystic mass, measuring approximately 8 cm in diameter, with heterogeneous enhancement surrounding the rim calcification in the pancreatic head area (Fig. 2). MRI showed low signal intensity on the T1-weighted images and high signal intensity on the T2-weighted images. The solid portion of the mixed mass showed homogenous delayed enhancement on the gadolinium- enhanced dynamic scan (Fig. 3). Fluorodeoxyglucose (FDG)-PET showed an irregular mass with increased FDG uptake (5.94 SUVmax) and hypermetabolic lymph nodes in the superior mesenteric artery (SMA) root area (Fig. 4). CT and MRI of the pancreas initially showed no lymph node enlargement, but the enlarged lymph node of the SMA root area was detected during the review of the pancreas CT after PET-CT. The preoperative diagnosis was a solid pseudopapillary neoplasm, and it is believed to be treatable by a complete resection and curative lymph node dissection. The patient underwent a pylorus-preserving pancreaticoduodenectomy because the main mass adhered to the retroperitoneum. Grossly, the specimen was a yellowish gray, well-circumscribed round mass (7.4×6.2 cm) with a granular cut surface. The metastatic lymph node of the SMA root area on FDG-PET/CT could not be dissected because it was encased in the SMA. The surgical resection margins of the tumor were clear. Microscopically, the tumor was composed of monomorphic cells with eosinophilic cytoplasm. Solid tumor cell nests surround the cuff of blood vessels, so pseudopapillae were formed. A focal solid growth pattern was observed, and the stroma showed calcification and ossification foci. Immunohistochemically, the tumor cells were positive for beta-catenin, CD10, CD56, and progesterone receptor (Fig. 5). It was diagnosed pathologically as a SPN of the pancreas. A peripancreatic lymph node metastasis was confirmed pathologically (pN1). The tumor stage was pT3N1M0, as described by the American Joint Committee on Cancer. The pathology of the tumor did not show malignant features. However, we could not completely exclude a solid pseudopapillary carcinoma because of the unresectable lymph node of the SMA root area. After a multidisciplinary team meeting, the plan was to monitor the residual lymph node of the SMA root area closely, where surgical resection could not be performed without additional treatment, such as chemotherapy or radiation therapy. Surgical resection and adjuvant therapy are considered when a patient is suspected of having a metastasis or progression. Fortunately, the current patient showed no changes in the SMA root area and no evidence of recurrence during the follow-up period (14 months).
After being first described by Frantz in 1959, a solid pseudopapillary tumor of the pancreas had various names, such as papillary cystic tumor/neoplasm/carcinoma, solid and papillary epithelial neoplasm, papillary and solid neoplasm, papillary and cystic tumor, until being classified by the World Health Organization (WHO) in 1996. It was then reclassified as an SPN, a low-grade malignant neoplasm of the exocrine pancreas, by the WHO in 2010.4
SPN is a rare pancreatic neoplasm that mostly occurs in young females, ranging in age from 20 to 30 years. It is rare in males, with a male-to-female ratio of 1:9. Because estrogen receptors are mainly present in young females, previous studies have attempted to reveal the role of estrogen receptors in the pathogenesis of tumors.5 On the other hand, there is no evidence to support this theory.6 The origin of SPN of the pancreas is unclear.
The clinical features of SPN in the pancreas are usually nonspecific. Although the most common symptom is abdominal pain, various clinical symptoms (including asymptomatic symptoms) have been reported. In addition, the tumor markers usually are within the normal range. SPN can be found in the tail (35.9%) and head (34%) of the pancreas.
Contrast-enhanced CT, which is the most helpful diagnostic examination, revealed a well-capsulated heterogeneous mass with mixed solid and cystic components. MRI revealed low to heterogeneous signal intensity on the T1-weighted images, heterogeneous to high signal intensity on the T2-weighted images and early heterogeneous and slowly progressive enhancement on the gadolinium-enhanced dynamic scan. Some studies on FDG-PET/CT of SPNs reported that FDG uptake varies from zero to high intensity.7 Recently, Park et al.8 reported how to analyze the metabolic characteristics of SPNs based on FDG uptake. They recommended that SPNs with a high maximum standardized uptake value (SUVmax) related to an increased Ki-67 index should be treated aggressively.8
The radiology findings of SPNs are helpful for diagnosis and differentiation from other pancreatic tumors. If the diagnosis remains unclear after CT or MRI, SPNs require a biopsy and cytology examination for an accurate preoperative diagnosis. In this respect, EUS-guided fine-needle aspiration (FNA) is a valuable method.9 Immunohistochemically, SPNs are typically positive for vimentin, neuron-specific enolase, α-1-antitrypsin, CD10, CD56, progesterone receptors, and beta- catenin, and negative for chromogranin, epithelial membrane antigen, and cytokeratin.10 In the present case, EUS-FNA was not performed because the preoperative radiology evaluation was consistent with SPN. Furthermore, a complete surgical resection was planned because the patient had opted for surgery to remove the tumor.
Although SPNs commonly show indolent growth and benign behavior, malignant degeneration may occur. Malignant SPN occurs in 18.3% of adult patients.11 Several studies have tried to identify the predictive factors related to this atypical occurrence leading to a poor prognosis utilizing the radiology findings and the cytological and immunochemical features. The results suggested that unusual rapid progression and malignancy potential were associated with large tumor size, vascular or perineural invasion, Ki-67 positivity, significant cellular pleomorphism, nuclear atypia, increased mitotic activity, and increased β-catenin.12,13 On the other hand, the pool of data collected was too small to make a meaningful association of the factors with the malignant potential of SPN. The criteria for judging a malignancy in SPN have not been well established, and several studies have used different criteria to determine a malignancy. For example, the WHO defines malignant behavior as perineural invasion, angioinvasion, and deep invasion into the surrounding. Butte et al. define malignant criteria as a locally unresectable tumor with an invasion of portal/mesenteric vessels, metastatic disease, or recurrence after resection.14 The criteria for malignancy reported by Kim et al. only included cellular atypia.14,15 Based on this arbitrary criterion of malignancy, in the present patient with a lymphatic invasion and unresectable metastatic lymph node but no vascular invasion, it might be considered a carcinoma according to other criteria, even though it did not correspond precisely to the malignant behavior of the WHO. According to the current data, 3% of individuals with SPN experience recurrence after resection; most of them occurred within 5 years.16
In this case, the poor prognostic factors were the residual lymph node of the SMA root area and the large tumor size. On the other hand, the exact cutoff value of large tumor size was not consistent in previous studies; some studies showed that a tumor size of over 5 cm and some 8 cm in size indicated a poor prognosis.17,18 Moreover, only a few studies reported the effects of a regional lymph node invasion on the prognosis of SPN. The clinical significance of regional lymph node invasion has not been established.19 Considering these points, the multidisciplinary team meeting decided on life-long monitoring without adjuvant therapy.
Surgery is the best treatment for SPN. The 5-year survival rate of SPNs after appropriate surgical resection is approximately 95%. It has a favorable prognosis, even with advanced or metastatic disease, because of its mostly slow progression and good surgical outcome. A few case reports have shown that adjuvant therapy, such as radiotherapy and chemotherapy, could be beneficial.20-22 On the other hand, there are no definite recommendations regarding adjuvant therapy in SPN with a positive postoperative margin or residual disease. Similar to this patient, some studies reported the patient's outcome without postoperative adjuvant therapy. Shuja and Alkimawi23 reported a case of SPN with a benign clinical course but showed poor prognostic factors for tumor recurrence, such as perineural and probable angiolymphatic invasion, where a pancreaticoduodenectomy had been performed with no adjuvant therapy. Lee et al.24 reported that a 25-year old patient with SPN in the tail of the pancreas and peritoneal seeding rejected adjuvant chemotherapy after undergoing debulking surgery. Although debulking surgery and a left lateral sectionectomy for increased seeding nodules and liver metastasis, respectively, were performed at 13 years and 16 years after the initial operation, she was alive 20 years after the initial operation.24 Goh et al.25 presented two patients who underwent R1 resections for malignant SPN and remained disease-free for a follow-up period of 13 years and 10 months. The role of adjuvant therapy in the survival outcome has not been established; therefore, further research will be needed.
In this case report, the patient did not receive adjuvant therapy, even with a residual metastatic unresectable lymph node, and had a good prognosis until the time this manuscript was written. The metastatic unresectable lymph node of the SMA root remained unchanged on follow-up CT. The patient was planned to be followed up with a pancreas CT every 6 months for 5 years after the initial surgery. After that, a life-long follow-up evaluation will be conducted yearly. Radiotherapy will be considered if an increase in residual lymph nodes is detected, and if distant metastases are detected, a surgical resection would first be considered, followed by adjuvant therapy, including chemotherapy.
In conclusion, although patients with SPN of the pancreas require long-term postoperative follow-up because of its slow progression, surgical resection of the primary tumor can be considered a promising treatment choice for SPN, even in residual metastases.
REFERENCES
1. Huang HL, Shih SC, Chang WH, Wang TE, Chen MJ, Chan YJ. 2005; Solid-pseudopapillary tumor of the pancreas: clinical experience and literature review. World J Gastroenterol. 11:1403–1409. DOI: 10.3748/wjg.v11.i9.1403. PMID: 15761986. PMCID: PMC4250695.

2. Papavramidis T, Papavramidis S. 2005; Solid pseudopapillary tumors of the pancreas: review of 718 patients reported in English literature. J Am Coll Surg. 200:965–972. DOI: 10.1016/j.jamcollsurg.2005.02.011. PMID: 15922212.

3. Guo N, Zhou QB, Chen RF, et al. 2011; Diagnosis and surgical treatment of solid pseudopapillary neoplasm of the pancreas: analysis of 24 cases. Can J Surg. 54:368–374. DOI: 10.1503/cjs.011810. PMID: 21939604. PMCID: PMC3238340.

4. Bosman FT, Carneiro F, Hruban RH, Theise ND. 2010. WHO Classification of tumours of the digestive system. 4th ed. IARC Publication;Lyon:
5. Nishihara K, Tsuneyoshi M, Ohshima A, Yamaguchi K. 1993; Papillary cystic tumor of the pancreas. Is it a hormone-dependent neoplasm? Pathol Res Pract. 189:521–526. DOI: 10.1016/S0344-0338(11)80359-6. PMID: 8378176.
6. Machado MC, Machado MA, Bacchella T, Jukemura J, Almeida JL, Cunha JE. 2008; Solid pseudopapillary neoplasm of the pancreas: distinct patterns of onset, diagnosis, and prognosis for male versus female patients. Surgery. 143:29–34. DOI: 10.1016/j.surg.2007.07.030. PMID: 18154930.

7. Cantisani V, Mortele KJ, Levy A, et al. 2003; MR imaging features of solid pseudopapillary tumor of the pancreas in adult and pediatric patients. AJR Am J Roentgenol. 181:395–401. DOI: 10.2214/ajr.181.2.1810395. PMID: 12876017.

8. Park M, Hwang HK, Yun M, Lee WJ, Kim H, Kang CM. 2018; Metabolic characteristics of solid pseudopapillary neoplasms of the pancreas: their relationships with high intensity 18F-FDG PET images. Oncotarget. 9:12009–12019. DOI: 10.18632/oncotarget.23846. PMID: 29552289. PMCID: PMC5844725.

9. De Moura DTH, Coronel M, Ribeiro IB, et al. 2018; The importance of endoscopic ultrasound fine-needle aspiration in the diagnosis of solid pseudopapillary tumor of the pancreas: two case reports. J Med Case Rep. 12:107. DOI: 10.1186/s13256-018-1585-3. PMID: 29695287. PMCID: PMC5918898.

10. La Rosa S, Bongiovanni M. 2020; Pancreatic solid pseudopapillary neoplasm: key pathologic and genetic features. Arch Pathol Lab Med. 144:829–837. DOI: 10.5858/arpa.2019-0473-RA. PMID: 31958381.

11. Yang F, Yu X, Bao Y, Du Z, Jin C, Fu D. 2016; Prognostic value of Ki-67 in solid pseudopapillary tumor of the pancreas: Huashan experience and systematic review of the literature. Surgery. 159:1023–1031. DOI: 10.1016/j.surg.2015.10.018. PMID: 26619927.

12. Yang F, Jin C, Long J, et al. 2009; Solid pseudopapillary tumor of the pancreas: a case series of 26 consecutive patients. Am J Surg. 198:210–215. DOI: 10.1016/j.amjsurg.2008.07.062. PMID: 19268906.

13. Tipton SG, Smyrk TC, Sarr MG, Thompson GB. 2006; Malignant potential of solid pseudopapillary neoplasm of the pancreas. Br J Surg. 93:733–737. DOI: 10.1002/bjs.5334. PMID: 16609955.

14. You L, Yang F, Fu DL. 2018; Prediction of malignancy and adverse outcome of solid pseudopapillary tumor of the pancreas. World J Gastrointest Oncol. 10:184–193. DOI: 10.4251/wjgo.v10.i7.184. PMID: 30079144. PMCID: PMC6068856.

15. Kim MJ, Choi DW, Choi SH, Heo JS, Sung JY. 2014; Surgical treatment of solid pseudopapillary neoplasms of the pancreas and risk factors for malignancy. Br J Surg. 101:1266–1271. DOI: 10.1002/bjs.9577. PMID: 25052300.

16. Rathi J, Anuragi G, J R LJ, R P, C S, O L NB. 2021; Prediction of recurrence risk in solid pseudopapillary neoplasm of the pancreas: single-institution experience. Cureus. 13:e17541. DOI: 10.7759/cureus.17541. PMID: 34646598. PMCID: PMC8478690.

17. Kang CM, Kim KS, Choi JS, Kim H, Lee WJ, Kim BR. 2006; Solid pseudopapillary tumor of the pancreas suggesting malignant potential. Pancreas. 32:276–280. DOI: 10.1097/01.mpa.0000202956.41106.8a. PMID: 16628083.

18. Kang CM, Choi SH, Kim SC, et al. 2014; Predicting recurrence of pancreatic solid pseudopapillary tumors after surgical resection: a multicenter analysis in Korea. Ann Surg. 260:348–355. DOI: 10.1097/SLA.0000000000000583. PMID: 24743622.

19. Lee G, Sung YN, Kim SJ, et al. 2021; Large tumor size, lymphovascular invasion, and synchronous metastasis are associated with the recurrence of solid pseudopapillary neoplasms of the pancreas. HPB (Oxford). 23:220–230. DOI: 10.1016/j.hpb.2020.05.015. PMID: 32654914.

20. Klöppel G, Solcia E, Sobin LH, Longnecker DS, Capella C. 1996. Histological typing of tumours of the exocrine pancreas. Springer Science & Business Media;Heidelberg: DOI: 10.1007/978-3-642-61024-0.
21. Strauss JF, Hirsch VJ, Rubey CN, Pollock M. 1993; Resection of a solid and papillary epithelial neoplasm of the pancreas following treatment with cis-platinum and 5-fluorouracil: a case report. Med Pediatr Oncol. 21:365–367. DOI: 10.1002/mpo.2950210511. PMID: 8492753.

22. Maffuz A, Bustamante Fde T, Silva JA, Torres-Vargas S. 2005; Preoperative gemcitabine for unresectable, solid pseudopapillary tumour of the pancreas. Lancet Oncol. 6:185–186. DOI: 10.1016/S1470-2045(05)01770-5. PMID: 15737835.

23. Shuja A, Alkimawi KA. 2014; Solid pseudopapillary tumor: a rare neoplasm of the pancreas. Gastroenterol Rep (Oxf). 2:145–149. DOI: 10.1093/gastro/gou006. PMID: 24759340. PMCID: PMC4020125.

24. Lee SE, Jang JY, Hwang DW, Park KW, Kim SW. 2008; Clinical features and outcome of solid pseudopapillary neoplasm: differences between adults and children. Arch Surg. 143:1218–1221. DOI: 10.1001/archsurg.143.12.1218. PMID: 19075175.

25. Goh BK, Tan YM, Cheow PC, et al. 2007; Solid pseudopapillary neoplasms of the pancreas: an updated experience. J Surg Oncol. 95:640–644. DOI: 10.1002/jso.20735. PMID: 17477365.

Fig. 1
Abdomen ultrasound revealed a 6.2 cm sized, peripheral calcified soft tissue mass at the portocaval area.
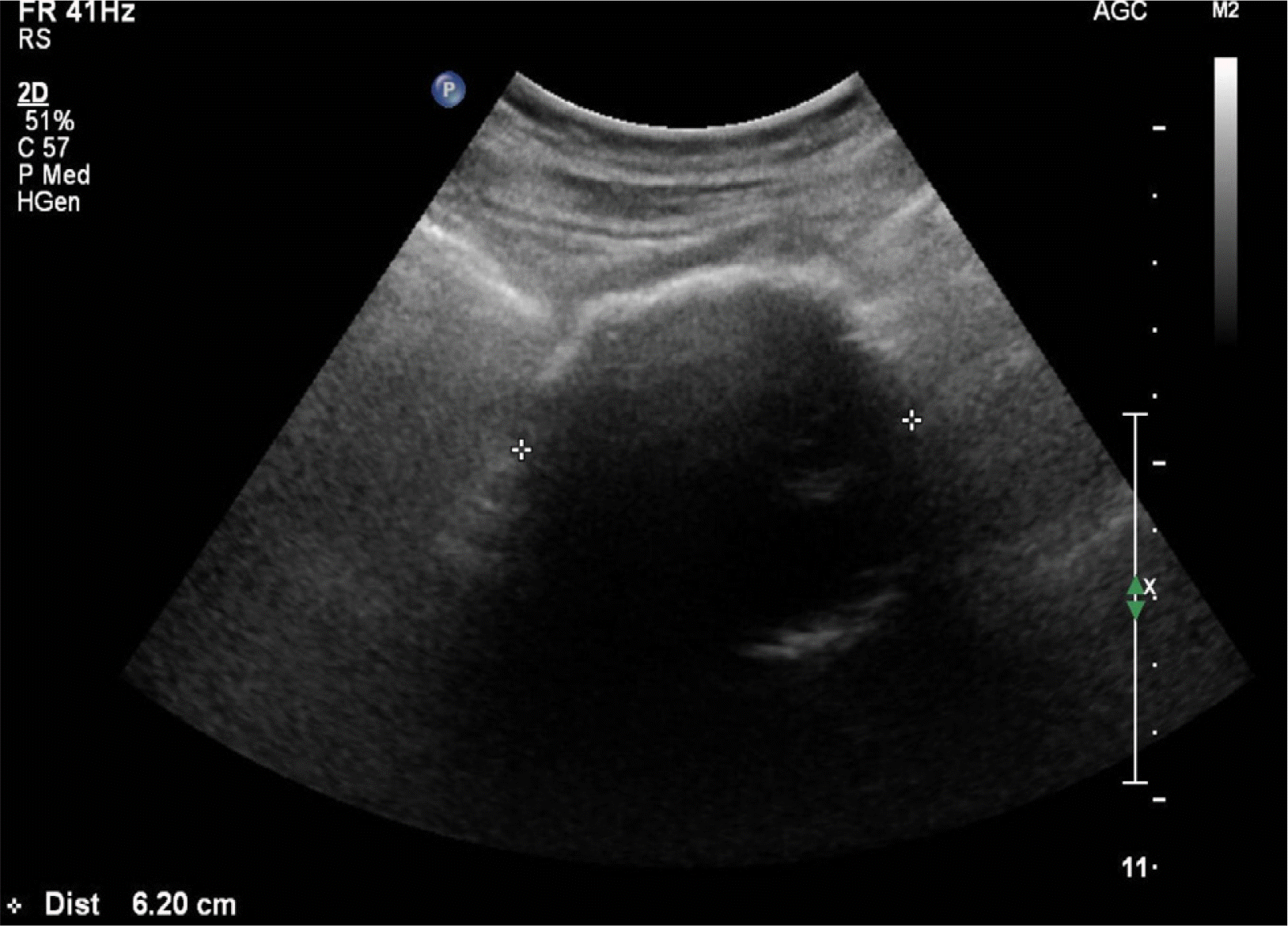
Fig. 2
Arterial phase of pancreas computed tomography revealed a mixed solid and cystic mass, with heterogeneous enhancement surrounding the rim calcification in the pancreatic head area or uncinate process.
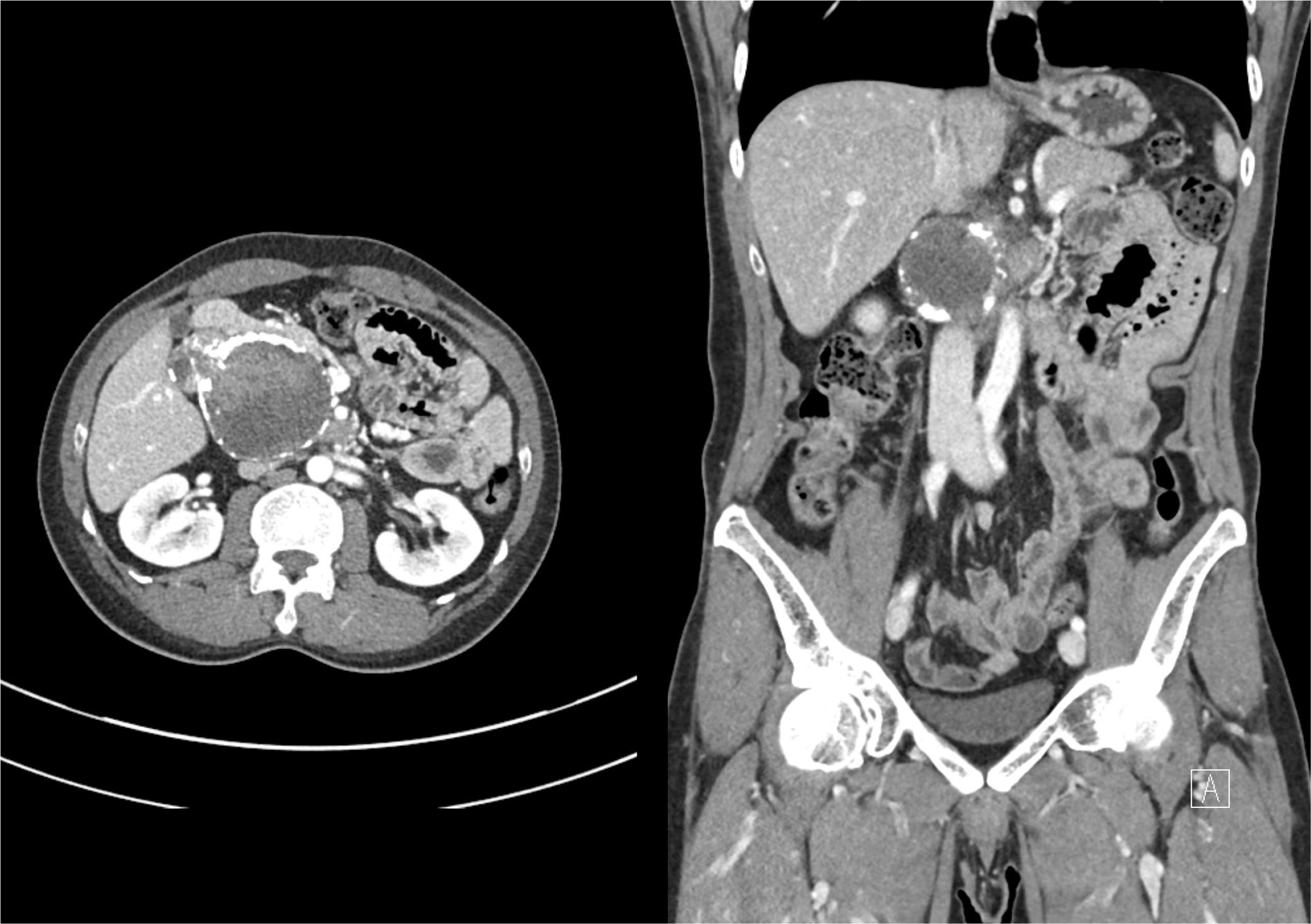
Fig. 3
Magnetic resonance imaging showed an 8 cm-sized mass with heterogeneous signal intensity. (A) It showed high signal intensity on T2-weighted images, (B) low signal intensity on T1-weighted images, and (C) a solid portion of it was showed relatively homogenous delayed enhancement on the gadolinium-enhanced dynamic scan.
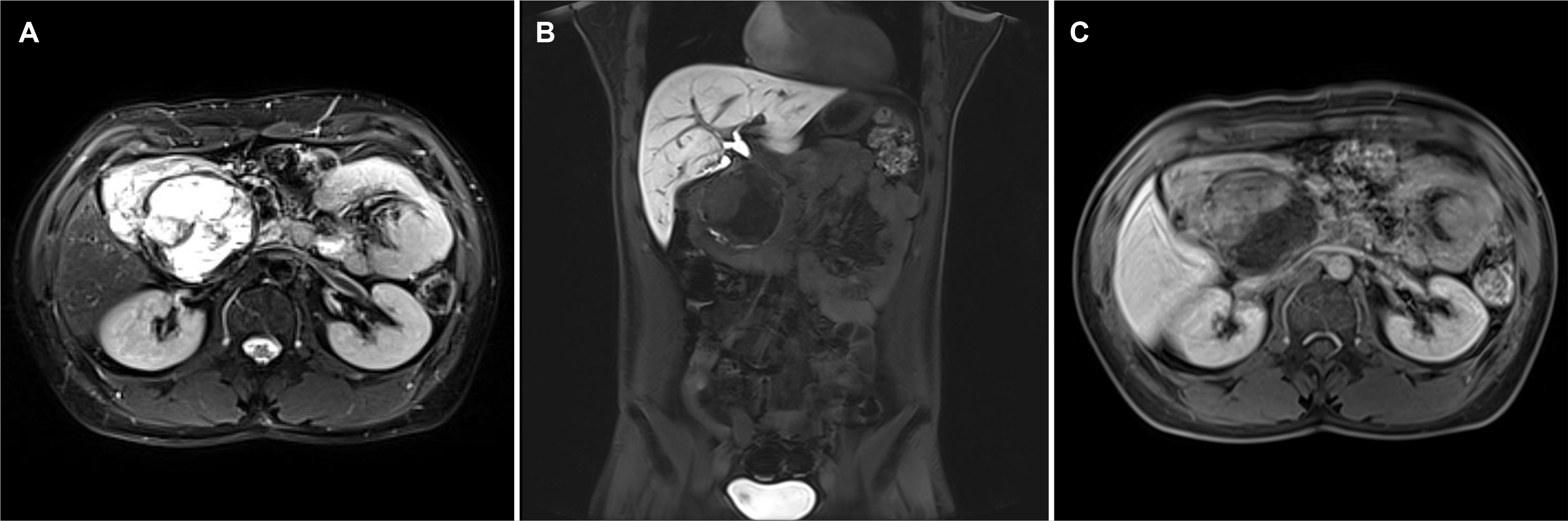
Fig. 4
Fluorodeoxyglucose-positron emission tomography/computed tomography scan showed a hypermetabolic mass (5.94 SUVmax) in the pancreas head area with uneven hypermetabolism. A metastatic lymph node in the superior mesenteric artery root area (yellow arrow) was revealed.
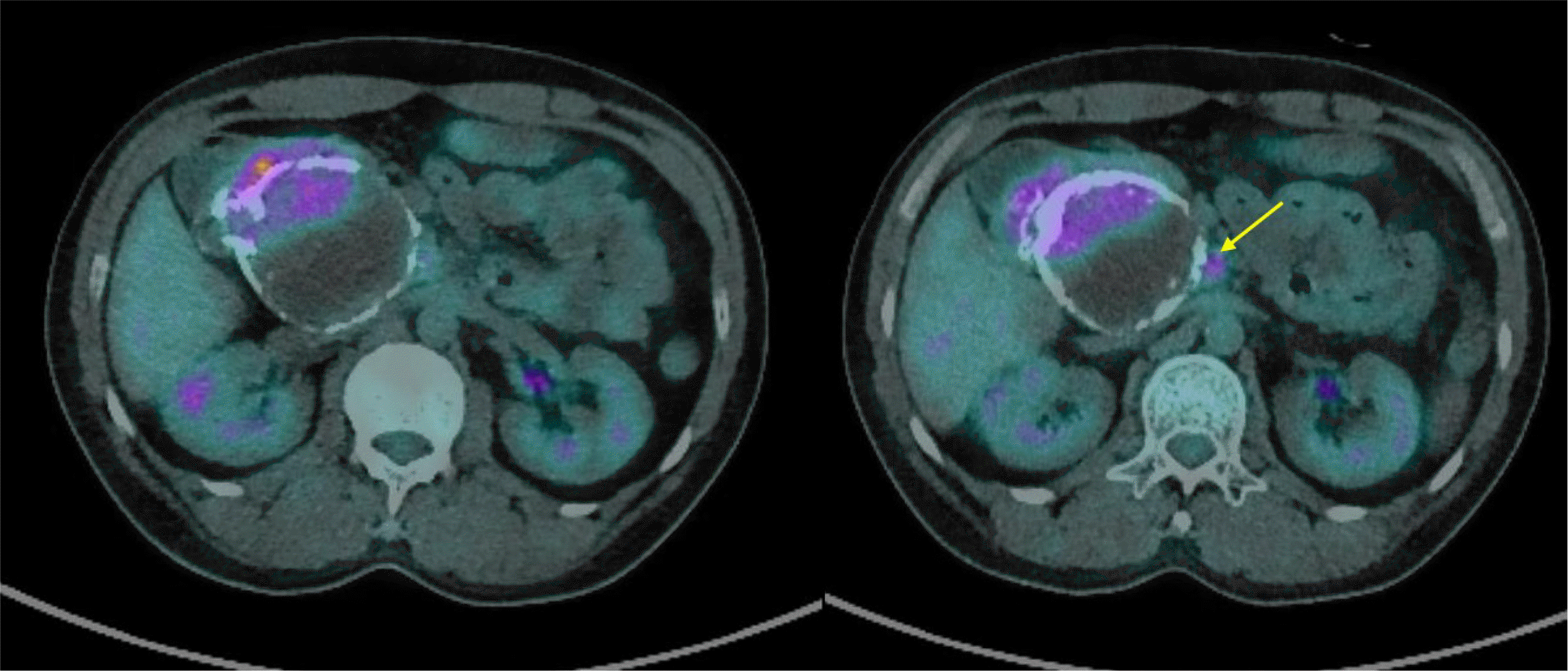
Fig. 5
Pathology findings. (A) The tumor showed pseudopapillary architecture (hematoxylin and eosin stain [H&E], ×100). (B) Focal solid growth pattern and hyaline globules were observed (H&E, ×100). (C) The tumor has multiple ossification foci surrounded by tumor cells (H&E, ×100). (D) The tumor cells showed nuclear and cytoplasmic positivity for beta-catenin (immunohistochemical stain, ×200).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download