Abstract
The lifetime incidence of epistaxis in dental and maxillofacial practice has been reported to be as high as 60% and can be caused by dental implant placement, Le Fort I osteotomy, intranasal supernumerary tooth, odontogenic tumors, blood disorders and maxillofacial trauma. Most epistaxis cases are minor and easily managed with direct compression on the nares for 10 minutes. For more significant or recurrent epistaxis, other techniques might include electrocautery, anterior or posterior nasal packing, or Foley catheter balloon. For patients with refractory epistaxis, cauterization of the sphenopalatine artery under endonasal endoscopy or embolization of the internal maxillary artery should be performed. Epistaxis control is required in patients diagnosed with inherited or acquired bleeding disorders or with drug-induced coagulopathies during dental procedures. In these cases, hemostatic system adjustment and hemostasis achieved by local and adjunctive methods are required. Dentists and maxillofacial surgeons must be aware that the nasal cavity is a potential source of perioperative hemorrhage. Depending on the invasiveness of the dental intervention, preoperative involvement of the hematologist and cardiologist is usually necessary to reverse anticoagulation or to cease anticoagulant therapy.
Epistaxis is a relatively frequent symptom, with a lifetime prevalence of approximately 60% in the general population1. Among postoperative complications, epistaxis following dental or maxillofacial surgery is uncommon2. However, due potential significant morbidity, dentists should be aware of the potential causes of epistaxis related to dental and maxillofacial procedures and be informed about basic treatments.
Most epistaxis cases (~90%) originate from the anterior nasal septum, supplied by Kiesselbach’s plexus3. These are branches of the internal maxillary artery, the sphenopalatine artery, and the anterior ethmoidal artery. The posterior nasal septum and the lateral nasal wall receive their vascular supply from the sphenopalatine artery and terminal branches of the internal maxillary artery3, which are the sources of posterior nasal bleeding.
The literature on epistaxis and its association with dental and maxillofacial interventions is limited. Thus, we reviewed specific management and treatment modalities to help dentists make decisions for such cases.
A literature search of PubMed, Embase, CENTRAL, and Cochrane Library databases was performed, focusing on studies published until April 2020. We combined the MeSH (Medical Subject Headings) terms “epistaxis,” “nose bleeding,” and “hemorrhage” with “dental interventions,” “dental implants,” “'Le Fort osteotomy,” “supernumerary tooth,” “blood disorders,” “odontogenic tumors,” and “maxillofacial trauma.” Results were checked for duplicates, and titles, abstracts, and full texts were evaluated to exclude irrelevant articles. We finally included 48 studies in our review. Studies were included regardless of type (review articles, original research, case reports). The inclusion criteria were determined according to the scope of study and data analysis: articles analyzing the diagnosis, source, and treatment approaches of epistaxis; etiology of nasal hemorrhage and selection of types of interventions for the sinonasal and oral areas; no gender or age restrictions. Article exclusion criteria were finite element analysis studies, animal studies, and in vitro studies.
The edentulous posterior maxillary is a challenging region for implant placement due to pneumatization of the maxillary sinus cavity, which can lead to lack of cortical bone height and resorption of the alveolar ridge4. The thick cortical bone of the maxillary sinus prevents direct spread of odontogenic infections into the maxilla bone. Nevertheless, the alveolar bone can become thinner due to aging, leaving only the Schneiderian membrane between the sinus and the mouth5. In this context, perforation of the Schneiderian membrane remains a potential complication during implant surgeries in approximately 15.7% of cases6, and bleeding in the maxilla can appear as epistaxis.
Bleeding in the maxilla is more likely when the bone covering the tooth roots is thin7,8; in cases of extensive sinus pneumatization, the alveolar process thins, exposing the alveolar canal with vessels to stimulating forces, such as dental implant surgery, with risk of massive bleeding8. Epistaxis was not a frequently reported complication after dental implant surgery (8.8% of cases).(Table 1) Epistaxis is more specifically observed when dentists place a part of a dental implant into the maxillary sinus to increase the height of the crestal bone9-11.(Table 1) In these studies, patients experienced self-limiting nose bleeds during the immediate postoperative period. However, Hong and Mun8 reported a case of dental implants in which a 54-year-old female patient appeared with serious nasal bleeding; the bleeding was so perfuse despite anterior and posterior nasal packing that the woman was eventually treated under general anesthesia. It is possible that the cause of the bleeding was injury to the posterior superior alveolar artery (Fig. 1) because the dental implant seemed to stick out toward the maxillary sinus, which was covered by Schneiderian’s membrane. Similarly, in their study, Bae et al.12 placed dental implants simultaneous with sinus floor elevation via either an osteotome or a reamer, and they recorded epistaxis as a complication in 6.7% (osteotome) and 5% (reamer) of patients, all of which resolved in the following days.(Table 1)
The Le Fort I maxillary osteotomy is a common maxillofacial surgery used to fix many malocclusion and maxillofacial abnormalities. Complications are rare, but epistaxis can occur when the pterygoid plates are separated from the maxilla. The involved vessels usually include the terminal branches of the internal maxillary artery, especially the descending palatine or sphenopalatine arteries, the posterior superior alveolar artery, and the pterygoid venous plexus13,14. The descending palatine artery and the posterior superior alveolar artery (Fig. 1) can be injured during Le Fort I osteotomy because of their location. Severing the posterior superior alveolar artery usually does not result in remarkable hemorrhaging unless it is a large-diameter vessel13. The descending palatine artery is more susceptible to damage during Le Fort I osteotomy because there is a possibility of vessel splitting when the surgeon separates the pterygomaxillary or the nasal wall14. Mehra et al.14 reported that the descending palatine artery is most likely to bleed after surgically-assisted rapid palatal expansion (SARPE) because this artery is injured by bone fractures.
Primary sources of epistaxis after Le Fort I osteotomies are the anterior, posterior, or both parts of the nasal cavity, and epistaxis usually occurs within the first 10 days after surgery15-19.(Table 2) Intubation and removal of the nasal mucosa from the nasal floor require special manipulation to avoid an isolated anterior20. Usually, posterior damage of an artery leads to bleeding from both nares20. The bleeding is not very serious and usually lasts 1 to 2 days. However, recurrent epistaxis has been noted in patients 1 to 8 weeks after orthognathic surgery, in which the presence of pseudoaneurysm was suspected during orthognathic interventions18,19,21, wherein an incomplete injury to one or more vessel-wall layers causes hemorrhaging in the surrounding tissue, leading to hematoma formation. Subsequent expansion of a hematoma due to arterial pressure can result in increased leakage and rupture19,21. According to a review22 of 19 patients with ruptured pseudoaneurysms following Le Fort I osteotomy, initial nasal bleeding began an average of 17.3±14.3 days (range, 3-62 days) following surgery. The majority of patients (67%) had multiple episodes of epistaxis after an initial period of minimal and self-limited epistaxis22. The affected vessels were the internal maxillary artery (38.9%), descending palatine artery (16.7%), sphenopalatine artery (38.9%), and internal carotid artery (5.6%)22.
Intranasal tooth eruption is an uncommon event, with incidence from 0.1% to 1% in the general population23. This can be either an extra tooth or a diversion of normal dentition (deciduous/permanent). Intranasal teeth present mainly in children, and are often asymptomatic, but can cause a variety of symptoms (nasal discharge, unilateral nasal obstruction, foul smell, anosmia, headaches), including recurrent epistaxis24. Epistaxis was reported to occur in young patients once or twice a month for two25 or three years26 during the period of tooth eruption into the nasal cavity.
Supernumerary nasal teeth are easily recognized from their small crowns in contrast with the root and the conical or tuberculate shape27. They grow in a vertical, horizontal, or inverted position, and they have been observed in the palate as extra teeth or can grow into the nasal cavity27. No hypotheses about their eruption are widely accepted. One view highlights faults during the interactions between the epithelia and mesenchym. There are also scientists who emphasize errors during the migration of neural crest derivatives to reach the jaw bones25.
Clinically, intranasal teeth present as an ivory white mass most often found on the floor of the nose, relatively anteriorly inside the nostril23,28; sometimes, intranasal teeth can be completely covered by nasal mucosa or granulation tissue24,25. Intranasal teeth can be found accidentally as a dense radiopaque shadow on panoramic radiograph24; however, computed tomography (CT) is more useful for showing supernumerary intranasal teeth26. Clinicians should distinguish an ectopic nasal tooth from foreign bodies, odontomas, rhinoliths, granulomatous infections, and tumors28.
Milam and Copper29 reported a case of prolonged epistaxis after dental extractions of the upper first and second molars in a 50-year-old patient with history of chronic hemodialysis. During surgery, an oroantral fistula was noted. Thus, a mucoperiosteal flap was raised, and good hemostasis was achieved. Thirty minutes after surgery, epistaxis from the right nostril and the right puncta developed after the maxillary sinus filled with blood. Within 24 hours, epistaxis subsided following nasal packing, without further incidence. Increased activity of prostaglandin (prostacyclin) with platelet antiaggregatory properties following hemodialysis was possibly responsible for epistaxis onset29.
However, epistaxis can exist prior to dental procedures, and dentists should know the effects of bleeding disorders on patient management, especially in invasive dental treatments. Bleeding disorders such as vascular defects (scurvy, purpura, hereditary hemorrhagic telangiectasia [Rendu–Osler–Weber syndrome]), platelet defects (quantitative or qualitative), or coagulation disorders (inherited or acquired, such as hemophilia A or B and Willebrand’s disease) are conditions with high risk of postoperative bleeding30. To prevent an episode of epistaxis, the medical history of each patient should be reviewed. Patients also might have undiagnosed hemostasis problems. Clinicians should ask their patients about bleeding episodes after surgeries or injuries, impulsive bleeding, or serious bleeding after dental extractions. Dentists must know the patient’s family history and their drug list (anticoagulants or antiplatelet therapy). Clinical manifestations of inherited bleeding disorders (e.g., hemophilia) are multiple purpura or ecchymoses of the skin, muscle (hematomas), or joints (hemarthrosis) and gingival or wound bleeds30,31. Blood counts and clotting tests (platelet count, prothrombin times) should also be carried out.
Very few case reports for odontogenic tumors have referred to epistaxis as a symptom. Virupakshappa et al.32 described an adenomatoid odontogenic tumor in a 20-year-old female patient who appeared with left epistaxis, associated with slow, progressive facial swelling and pain on the left half of her face. After surgical management of the tumor, swelling and epistaxis resolved. Ameloblastoma, an aggressive neoplasm in the mandible or maxilla, was reported for a 61-year-old white man presenting with unilateral nasal obstruction with long-term epistaxis after extension of the tumor into the nose33. The patient was treated with removal of the tumor and the medial wall of the maxilla.
In severe midface trauma, epistaxis usually occurs immediately after facial injury, including Le Fort II and III fractures34. Nasal bone fractures usually result in minor epistaxis, which resolves spontaneously. Trauma to the nasoethmoidal complex could be responsible for severe and persistent epistaxis35. Epistaxis caused by intrusive luxation of the permanent teeth, such as an upper maxillary tooth into the nasal cavity, is rare36. An 11-year-old male injured his maxilla during a fall36, and a fracture of the maxillary alveolar bone with an intrusive luxation of the right central incisor was found. The injury penetrated the floor of the nasal cavity causing epistaxis. Under local anesthesia, surgical (not orthodontic) repositioning of the incisor and bone segment was performed, with subsequent fixation using titanium micromesh and screws. No serious symptoms were noticed during a 1.5-year follow-up36.
Epistaxis treatment varies according to bleeding severity and type. If the bleeding is minor, the patient should lean forward to reduce blood swallowing or aspiration and continuously hold pressure on the nares to the cartilaginous portion of the nose just below the nasal bones for 10 minutes to achieve hemostasis. If bleeding persists or is more serious, the source site should be identified to determine whether the epistaxis is anterior or posterior. For anterior epistaxis, electrocautery is recommended directly on the anterior part of the nasal septum, which contains the Kiesselbach’s plexus of vessels. When cautery fails to achieve adequate hemostasis, anterior nasal packing will need to be inserted (Fig. 2) and remain in place for 48 hours1,3. Posterior epistaxis is suspected when the bleeding is excessive, bilateral, or accompanied by hematemesis or hemoptysis. Posterior nasal packing is indicated using a Foley catheter balloon (Fig. 3), which is inserted under local anesthesia on the bleeding side, while anterior packing is performed on the contralateral side. The Foley catheter is inflated with up to 10 mL of normal saline until no blood is observed in the pharynx. Administration of systemic antibiotics is recommended while anterior and posterior packing are maintained3,37. In cases of severe intractable epistaxis with repeated failure of packing insertions, cauterization-clipping of the sphenopalatine artery following endonasal endoscopy is performed under general anesthesia38, at which time, bleeding sites can be cauterized, spurs can be removed, and a deviated septum can be straightened. Another valuable tool to control severe epistaxis is angiography, which involves embolization of the internal maxillary artery (Fig. 1) and, in selected cases, the facial artery. With this technique, under general anesthesia and systemic heparinization, the femoral artery is punctured, and a catheter is used to catheterize both external carotid arteries and internal maxillary arteries. These vessels, which are responsible for epistaxis, are then embolized with microparticles (300-500 μm) with success rates up to 97%39.
Remarkable bleeding is often reported after implant placement, mainly in the mandible40. During therapeutic management, early attention should focus on evacuating local hematomas, identifying the damaged artery, and controlling the hemorrhage either with ligation or vessel cauterization. For cases where concomitant epistaxis is not conservatively controlled, a surgical technique involving maxillary sinus osteoplasty with a vascularized pedicle bone flap through a maxillary sinus approach has been reported8, wherein a horizontal incision in the upper vestibule was performed in front of the maxillary wall, a mucoperiosteal flap was raised, and a bony cut toward the inferior maxillary wall was made to provide access to the dental implant41. The bleeding site from the surrounding mucosal membrane and blood vessel were identified and effectively coagulated8. This intraoral method can replace endoscopic endonasal surgery, which can be challenging because of the acute angle from the middle or inferior nasal meatus to the bottom of the maxillary sinus, resulting in reduced visibility42.
When epistaxis occurs after Le Fort I osteotomies, maxillomandibular fixation should be released, if used, to achieve a better intraoral view and to aspirate postnasal and pharyngeal clots. This allows the physician to estimate the degree of epistaxis severity. As in cases of active hemorrhage, vital signs should be monitored, an intravenous catheter should be placed13, and anterior and posterior nasal packing must be inserted into the nose to stop the bleeding. If postoperative epistaxis occurs early after surgery, a re-exploration should be considered; if bleeding occurs later or it is recurrent, angiography and subsequent embolization are indicated13. Mehra et al.14 reported a case of brisk epistaxis 11 days after a SARPE procedure, in which classical Le Fort I osteotomies were performed including pterygomaxillary separation without downfracturing the maxilla. Delayed epistaxis occurred but was managed successfully with posterior nasal packing via Foley catheters. The authors14 suggested surgical collapse of the expanded maxilla when adequate hemostasis was not achieved and epistaxis was not controlled after nasal packing because this could visualize the hemorrhage source in the maxilla and allow the pterygopalatine fossa to be tamponated more easily.
In a recent review43 of 23 studies, epistaxis was noted in six (22.2%) of 27 patients, in which all these patients underwent CT scans to determine the location of intranasal teeth. The main approaches for tooth removal are intraoral and transnasal. The intraoral approach can harm neighboring teeth and damage the neurovascular bundle and oronasal fistulae23. Extraction of the intranasal tooth under endoscopic guidance has advantages of good illumination, clear visualization, and precise dissection with retained neighboring structures28. Total resolution of epistaxis and other symptoms was reported at 24 months after surgery43.
Patients with hemophilia are at high risk of intra- and postoperative epistaxis following dental procedures. Increasing factor VIII level for hemophilia A (factor IX for hemophilia B) and replacing factor VIII and inhibiting fibrinolysis (tranexamic acid) for patients undergoing dental or maxillofacial surgery are required30. Mechanical compression with vasoconstrictors and local hemostatic agents, such as collagen vials (Helistat), cellulose-based products (Tampotamp, Surgicel), fibrin glue (Tissucol), and resorbable sutures44, can be locally applied. Desmopressin (DDAVP) is used to increase factor VIII and can also be administered in von Willebrand’s disease45.
For dental procedures, it is not necessary to modify antiplatelet therapy or vitamin K antagonists administered for primary or secondary cardiovascular disease prevention46. Moreover, novel oral anticoagulants have been developed to treat atrial fibrillation, pulmonary thromboembolism and venous thrombosis47. In a recent study47 of 153 cases prescribed novel oral anticoagulants, postoperative bleeding such as epistaxis occurred in only nine cases (two after scaling, three after simple extraction, three after first-stage implant surgery, one after resin filling). The authors reported that it is safe to perform most dental practices in patients taking these anticoagulants regardless of the anticoagulant's discontinuance duration; however, they postulated that dental and maxillofacial procedures requiring graft and osteotomy need at least 24 hours of discontinuance47. In cases of severe epistaxis, attempts to reverse anticoagulation or to cease anticoagulant therapy should be considered48. Idarucizumab is an antibody antigen-binding fragment that completely reverses the anticoagulant effect within minutes of administration48.
Gentle in-depth placement of bilateral anterior nasal packing with Vaseline gauze is recommended for epistaxis, and more aggressive placement of a Foley catheter balloon for posterior epistaxis should be avoided to not aggravate any preexisting traumatic tearing or laceration of the nasal mucosa. Patients with severe or recalcitrant epistaxis might need cauterization-clipping of the sphenopalatine artery or ethmoidal arteries after lateral canthotomy (Lynch incision)35. Angiography-embolization of the internal maxillary artery and ethmoidal arteries should be last options in cases of intractable epistaxis.
There have been many reported complications of intraoral bleeding episodes following dental interventions, but there is a lack of reviews assessing epistaxis resulting from dental and maxillofacial surgery. In our review, the main odontogenic causes leading to epistaxis were described, and general guidance for managing epistaxis was provided. The source of nasal bleeding can be located in the nasal cavity (intranasal supernumerary tooth, odontogenic tumor, nasal fracture), the maxillary sinus (dental implants), the oral cavity (dental procedures with blood disorders), or even the pterygomaxillary area (Le Fort osteotomy, maxillofacial trauma). However, epistaxis as an epiphenomenon should be treated in the same way regardless of bleeding site.
For slight epistaxis, the patient should be treated with rest, mild sedatives, and observation. Unless epistaxis stops, there is need for more definitive therapy, including nasal electrocautery or anterior and posterior nasal packing. Packing is not effective for major posterior epistaxis. At that point, depending on the location and extent of dental surgery, it is crucial to decide whether to reexplore the surgical site (after dental implant placement or Le Fort I osteotomy), especially if epistaxis occurs early in the postoperative period. Otherwise, invasive procedures, such as cauterization of the sphenopalatine artery under endonasal endoscopy or embolization of the internal maxillary artery, should be performed. It is widely debated whether endonasal cauterization-ligation of the sphenopalatine artery or embolization should be the first choice for intractable posterior epistaxis. In our center, we prefer sphenopalatine artery cauterization when epistaxis is not resolved by conservative treatment. However, for epistaxis after dental or maxillofacial procedures, such as dental implant and osteotomy procedures, angiography-embolization has the advantage of showing the hemorrhage site, allowing precise treatment (even if the patient is actively bleeding)2,14,22. Moreover, as angiographic localization and embolization can be carried out at the same time, it is valuable to pinpoint ruptured pseudoaneurysms of the internal maxillary artery and to expose the collateral blood flow contributing to epistaxis13,18. Embolization of the bilateral internal maxillary artery or the ipsilateral facial artery can be performed without complications and no significant recurrent epistaxis and can also be performed in cases of maxilla trauma, odontogenic tumors, vascular abnormalities, and bleeding diathesis39.
Generally, in patients with bleeding disorders such as vascular defects, it is important to reinforce hemostasis during dental or maxillofacial surgery using local measures based on mechanical compression, suturing, and hemostatic agents (Surgicel, Gelfoam, gauzes)31. With platelet defects, the minimum blood platelet level before dental procedures is 50,000/μL; for extensive procedures, it should be more than 100,000/μL. Replacement therapy with platelet transfusion might be required 30 minutes before surgery30. For patients with coagulation disorders, the missing factor should be administered prior to surgery45. Dentists need to be informed of the potential severity of epistaxis and the increased likelihood of recurrence in patients taking oral anticoagulation therapy who are undergoing dental or maxillofacial interventions. Preoperative involvement of the hematologist and cardiologist is usually necessary to modify drug dosage48.
Notes
Authors’ Contributions
G.P. participated in data collection and wrote the manuscript. G.P., G.G.D., and D.P. participated in the study design and performed the statistical analysis. C.S. and J.C. participated in the study design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
References
1. Morgan DJ, Kellerman R. 2014; Epistaxis: evaluation and treatment. Prim Care. 41:63–73. https://doi.org/10.1016/j.pop.2013.10.007. DOI: 10.1016/j.pop.2013.10.007. PMID: 24439881.

2. Solomons NB, Blumgart R. 1988; Severe late-onset epistaxis following Le Fort I osteotomy: angiographic localization and embolization. J Laryngol Otol. 102:260–3. https://doi.org/10.1017/s0022215100104670. DOI: 10.1017/S0022215100104670. PMID: 3356937.

3. Krulewitz NA, Fix ML. 2019; Epistaxis. Emerg Med Clin North Am. 37:29–39. https://doi.org/10.1016/j.emc.2018.09.005. DOI: 10.1016/j.emc.2018.09.005. PMID: 30454778.

4. Tatum H Jr. 1986; Maxillary and sinus implant reconstructions. Dent Clin North Am. 30:207–29. PMID: 3516738.
5. Ferguson M. 2014; Rhinosinusitis in oral medicine and dentistry. Aust Dent J. 59:289–95. https://doi.org/10.1111/adj.12193. DOI: 10.1111/adj.12193. PMID: 24861778.

6. Stacchi C, Andolsek F, Berton F, Perinetti G, Navarra CO, Di Lenarda R. 2017; Intraoperative complications during sinus floor elevation with lateral approach: a systematic review. Int J Oral Maxillofac Implants. 32:e107–18. https://doi.org/10.11607/jomi.4884. DOI: 10.11607/jomi.4884. PMID: 28494033.

7. Alberti PW. 1976; Applied surgical anatomy of the maxillary sinus. Otolaryngol Clin North Am. 9:3–20. DOI: 10.1016/S0030-6665(20)32713-4. PMID: 1246431.

8. Hong YH, Mun SK. 2011; A case of massive maxillary sinus bleeding after dental implant. Int J Oral Maxillofac Surg. 40:758–60. https://doi.org/10.1016/j.ijom.2010.11.027. DOI: 10.1016/j.ijom.2010.11.027. PMID: 21377328.

9. Nooh N. 2013; Effect of schneiderian membrane perforation on posterior maxillary implant survival. J Int Oral Health. 5:28–34. PMID: 24155599. PMCID: PMC3769876.
10. Kim HY, Yang JY, Chung BY, Kim JC, Yeo IS. 2013; Peri-implant bone length changes and survival rates of implants penetrating the sinus membrane at the posterior maxilla in patients with limited vertical bone height. J Periodontal Implant Sci. 43:58–63. https://doi.org/10.5051/jpis.2013.43.2.58. DOI: 10.5051/jpis.2013.43.2.58. PMID: 23678388. PMCID: PMC3651938.

11. Shihab OI. 2017; Intentional penetration of dental implants into the maxillary sinus: a retrospective study. Zanco J Med Sci. 21:1536–9. https://doi.org/10.15218/zjms.2017.001. DOI: 10.15218/zjms.2017.001.

12. Bae OY, Kim YS, Shin SY, Kim WK, Lee YK, Kim SH. 2015; Clinical outcomes of reamer- vs osteotome-mediated sinus floor elevation with simultaneous implant placement: a 2-year retrospective study. Int J Oral Maxillofac Implants. 30:925–30. https://doi.org/10.11607/jomi.3779. DOI: 10.11607/jomi.3779. PMID: 26252045.

13. Lanigan DT, Hey JH, West RA. 1990; Major vascular complications of orthognathic surgery: hemorrhage associated with Le Fort I osteotomies. J Oral Maxillofac Surg. 48:561–73. https://doi.org/10.1016/s0278-2391(10)80468-9. DOI: 10.1016/S0278-2391(10)80468-9. PMID: 2341936.

14. Mehra P, Cottrell DA, Caiazzo A, Lincoln R. 1999; Life-threatening, delayed epistaxis after surgically assisted rapid palatal expansion: a case report. J Oral Maxillofac Surg. 57:201–4. https://doi.org/10.1016/s0278-2391(99)90241-0. DOI: 10.1016/S0278-2391(99)90241-0. PMID: 9973133.

15. Williams BJ, Currimbhoy S, Silva A, O'Ryan FS. 2012; Complications following surgically assisted rapid palatal expansion: a retrospective cohort study. J Oral Maxillofac Surg. 70:2394–402. https://doi.org/10.1016/j.joms.2011.09.050. DOI: 10.1016/j.joms.2011.09.050. PMID: 22516838.

16. de Mol van Otterloo JJ, Tuinzing DB, Greebe RB, van der Kwast WA. 1991; Intra- and early postoperative complications of the Le Fort I osteotomy. A retrospective study on 410 cases. J Craniomaxillofac Surg. 19:217–22. https://doi.org/10.1016/s1010-5182(05)80551-7. DOI: 10.1016/S1010-5182(05)80551-7. PMID: 1894740.

17. Eshghpour M, Mianbandi V, Samieirad S. 2018; Intra- and postoperative complications of Le Fort I maxillary osteotomy. J Craniofac Surg. 29:e797–803. https://doi.org/10.1097/SCS.0000000000004828. DOI: 10.1097/SCS.0000000000004828. PMID: 30277955.

18. Avelar RL, Goelzer JG, Becker OE, de Oliveira RB, Raupp EF, de Magalhães PS. 2010; Embolization of pseudoaneurysm of the internal maxillary artery after orthognathic surgery. J Craniofac Surg. 21:1764–8. https://doi.org/10.1097/SCS.0b013e3181f40393. DOI: 10.1097/SCS.0b013e3181f40393. PMID: 21119417.

19. Park B, Jang WH, Lee BK. 2019; An idiopathic delayed maxillary hemorrhage after orthognathic surgery with Le Fort I osteotomy: a case report. J Korean Assoc Oral Maxillofac Surg. 45:364–8. https://doi.org/10.5125/jkaoms.2019.45.6.364. DOI: 10.5125/jkaoms.2019.45.6.364. PMID: 31966982. PMCID: PMC6955418.

20. Garg S, Kaur S. 2014; Evaluation of post-operative complication rate of Le Fort I osteotomy: a retrospective and prospective study. J Maxillofac Oral Surg. 13:120–7. https://doi.org/10.1007/s12663-012-0457-4. DOI: 10.1007/s12663-012-0457-4. PMID: 24822002. PMCID: PMC4016385.

21. Niazi MH, El-Ghanem M, Al-Mufti F, Wajswol E, Dodson V, Abdulrazzaq A, et al. 2018; Endovascular management of epistaxis secondary to dissecting pseudoaneurysm of the descending palatine artery following orthognathic surgery. J Vasc Interv Neurol. 10:41–6. PMID: 30746009. PMCID: PMC6350872.
22. Bykowski MR, Hill A, Garland C, Tobler W, Losee JE, Goldstein JA. 2018; Ruptured pseudoaneurysm of the maxillary artery and its branches following Le Fort I osteotomy: evidence-based guidelines. J Craniofac Surg. 29:998–1001. https://doi.org/10.1097/SCS.0000000000004396. DOI: 10.1097/SCS.0000000000004396. PMID: 29498976.

23. Krishnan B, Parida PK, Gopalakrishnan S, Satyparakash MV. 2013; An unusual cause of epistaxis in a young patient: the supernumerary nasal tooth. Oral Maxillofac Surg. 17:315–7. https://doi.org/10.1007/s10006-013-0393-6. DOI: 10.1007/s10006-013-0393-6. PMID: 23344617.

24. Iwai T, Aoki N, Yamashita Y, Omura S, Matsui Y, Maegawa J, et al. 2012; Endoscopic removal of bilateral supernumerary intranasal teeth. J Oral Maxillofac Surg. 70:1030–4. https://doi.org/10.1016/j.joms.2011.10.014. DOI: 10.1016/j.joms.2011.10.014. PMID: 22538022.

25. Verma RK, Bakshi J, Panda NK. 2012; Ectopic intranasal tooth: an unusual cause of epistaxis in a child. Ear Nose Throat J. 91:242–4. DOI: 10.1177/014556131209100606. PMID: 22711391.

26. Al Dhafeeri HO, Kavarodi A, Al Shaikh K, Bukhari A, Al Hussain O, El Baramawy A. 2014; Recurrent epistaxis caused by an intranasal supernumerary tooth in a young adult. Am J Case Rep. 15:291–3. https://doi.org/10.12659/AJCR.890710. DOI: 10.12659/AJCR.890710. PMID: 25031783. PMCID: PMC4099208.

27. Kirmeier R, Truschnegg A, Payer M, Malyk J, Daghighi S, Jakse N. 2009; The supernumerary nasal tooth. Int J Oral Maxillofac Surg. 38:1219–22. https://doi.org/10.1016/j.ijom.2009.06.013. DOI: 10.1016/j.ijom.2009.06.013. PMID: 19596556.

28. Lee FP. 2001; Endoscopic extraction of an intranasal tooth: a review of 13 cases. Laryngoscope. 111:1027–31. https://doi.org/10.1097/00005537-200106000-00017. DOI: 10.1097/00005537-200106000-00017. PMID: 11404615.

29. Milam SB, Cooper RL. 1983; Extensive bleeding following extractions in a patient undergoing chronic hemodialysis. Oral Surg Oral Med Oral Pathol. 55:14–6. https://doi.org/10.1016/0030-4220(83)90298-0. DOI: 10.1016/0030-4220(83)90298-0. PMID: 6600828.

30. Gupta A, Epstein JB, Cabay RJ. 2007; Bleeding disorders of importance in dental care and related patient management. J Can Dent Assoc. 73:77–83. PMID: 17295950.
31. Rafique S, Fiske J, Palmer G, Daly B. 2013; Special care dentistry: part 1. Dental management of patients with inherited bleeding disorders. Dent Update. 40:613–6. 619–22. 625–6 passim. https://doi.org/10.12968/denu.2013.40.8.613. DOI: 10.12968/denu.2013.40.8.613. PMID: 24279214.

32. Virupakshappa D, Rajashekhara BS, Manjunatha BS, Das N. 2014; Adenomatoid odontogenic tumour in a 20-year-old woman. BMJ Case Rep. 2014:bcr2013010436. https://doi.org/10.1136/bcr-2013-010436. DOI: 10.1136/bcr-2013-010436. PMID: 24810436. PMCID: PMC3992600.

33. Wenig BL, Sciubba JJ, Cohen A, Goldstein A, Abramson AL. 1985; An unusual cause of unilateral nasal obstruction: ameloblastoma. Otolaryngol Head Neck Surg. 93:426–32. https://doi.org/10.1177/019459988509300327. DOI: 10.1177/019459988509300327. PMID: 3927244.

34. Kühnel TS, Reichert TE. 2015; Trauma of the midface. GMS Curr Top Otorhinolaryngol Head Neck Surg. 14:Doc06. https://doi.org/10.3205/cto000121. DOI: 10.3205/cto000121. PMID: 26770280. PMCID: PMC4702055.

35. Dallan I, Tschabitscher M, Castelnuovo P, Bignami M, Muscatello L, Lenzi R, et al. 2009; Management of severely bleeding ethmoidal arteries. J Craniofac Surg. 20:450–4. https://doi.org/10.1097/SCS.0b013e31819b9803. DOI: 10.1097/SCS.0b013e31819b9803. PMID: 19276822.

36. Yonezawa H, Yanamoto S, Hoshino T, Yamada S, Fujiwara T, Umeda M. 2013; Management of maxillary alveolar bone fracture and severely intruded maxillary central incisor: report of a case. Dent Traumatol. 29:416–9. https://doi.org/10.1111/j.1600-9657.2011.01111.x. DOI: 10.1111/j.1600-9657.2011.01111.x. PMID: 22233224.

37. Pashen D, Stevens M. 2002; Management of epistaxis in general practice. Aust Fam Physician. 31:717–21. PMID: 12189661.
38. Kitamura T, Takenaka Y, Takeda K, Oya R, Ashida N, Shimizu K, et al. 2019; Sphenopalatine artery surgery for refractory idiopathic epistaxis: systematic review and meta-analysis. Laryngoscope. 129:1731–6. https://doi.org/10.1002/lary.27767. DOI: 10.1002/lary.27767. PMID: 30613985.

39. Shah QA. 2008; Bilateral tri-arterial embolization for the treatment of epistaxis. J Vasc Interv Neurol. 1:102–5. PMID: 22518233. PMCID: PMC3317326.
40. Tomljenovic B, Herrmann S, Filippi A, Kühl S. 2016; Life-threatening hemorrhage associated with dental implant surgery: a review of the literature. Clin Oral Implants Res. 27:1079–84. https://doi.org/10.1111/clr.12685. DOI: 10.1111/clr.12685. PMID: 26364684.

41. Lazaridis N, Tilaveridis I, Venetis G, Lazaridou M. 2008; Maxillary sinus osteoplasty with vascularized pedicled bone flap. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 106:828–32. https://doi.org/10.1016/j.tripleo.2008.05.030. DOI: 10.1016/j.tripleo.2008.05.030. PMID: 18656389.

42. Aukštakalnis R, Simonavičiūtė R, Simuntis R. 2018; Treatment options for odontogenic maxillary sinusitis: a review. Stomatologija. 20:22–6. PMID: 29806655.
43. Levin M, Sommer DD. 2019; Endoscopic removal of ectopic sinonasal teeth: a systematic review. J Otolaryngol Head Neck Surg. 48:30. https://doi.org/10.1186/s40463-019-0353-8. DOI: 10.1186/s40463-019-0353-8. PMID: 31277707. PMCID: PMC6612209.

44. Peisker A, Raschke GF, Schultze-Mosgau S. 2014; Management of dental extraction in patients with haemophilia A and B: a report of 58 extractions. Med Oral Patol Oral Cir Bucal. 19:e55–60. https://doi.org/10.4317/medoral.19191. DOI: 10.4317/medoral.19191. PMID: 24121912. PMCID: PMC3909433.

45. Anderson JA, Brewer A, Creagh D, Hook S, Mainwaring J, McKernan A, et al. 2013; Guidance on the dental management of patients with haemophilia and congenital bleeding disorders. Br Dent J. 215:497–504. https://doi.org/10.1038/sj.bdj.2013.1097. DOI: 10.1038/sj.bdj.2013.1097. PMID: 24264665.

46. Dézsi CA, Dézsi BB, Dézsi AD. 2017; Management of dental patients receiving antiplatelet therapy or chronic oral anticoagulation: a review of the latest evidence. Eur J Gen Pract. 23:196–201. Erratum in: Eur J Gen Pract 2017;23:i-ii. https://doi.org/10.1080/13814788.2017.1350645. DOI: 10.1080/13814788.2017.1350645. PMID: 28743214. PMCID: PMC5774272.

47. Kwak EJ, Nam S, Park KM, Kim SY, Huh J, Park W. 2019; Bleeding related to dental treatment in patients taking novel oral anticoagulants (NOACs): a retrospective study. Clin Oral Investig. 23:477–84. https://doi.org/10.1007/s00784-018-2458-2. DOI: 10.1007/s00784-018-2458-2. PMID: 29696419.

48. Ho JPK, Bari N, Riffat F. 2020; Management of epistaxis in patients on novel oral anticoagulation therapy. J Laryngol Otol. 134:316–22. https://doi.org/10.1017/S0022215120000754. DOI: 10.1017/S0022215120000754. PMID: 32281535.

Fig. 1
Anatomical diagram of the internal maxillary artery and its branches (sources of posterior epistaxis). (a.: artery)
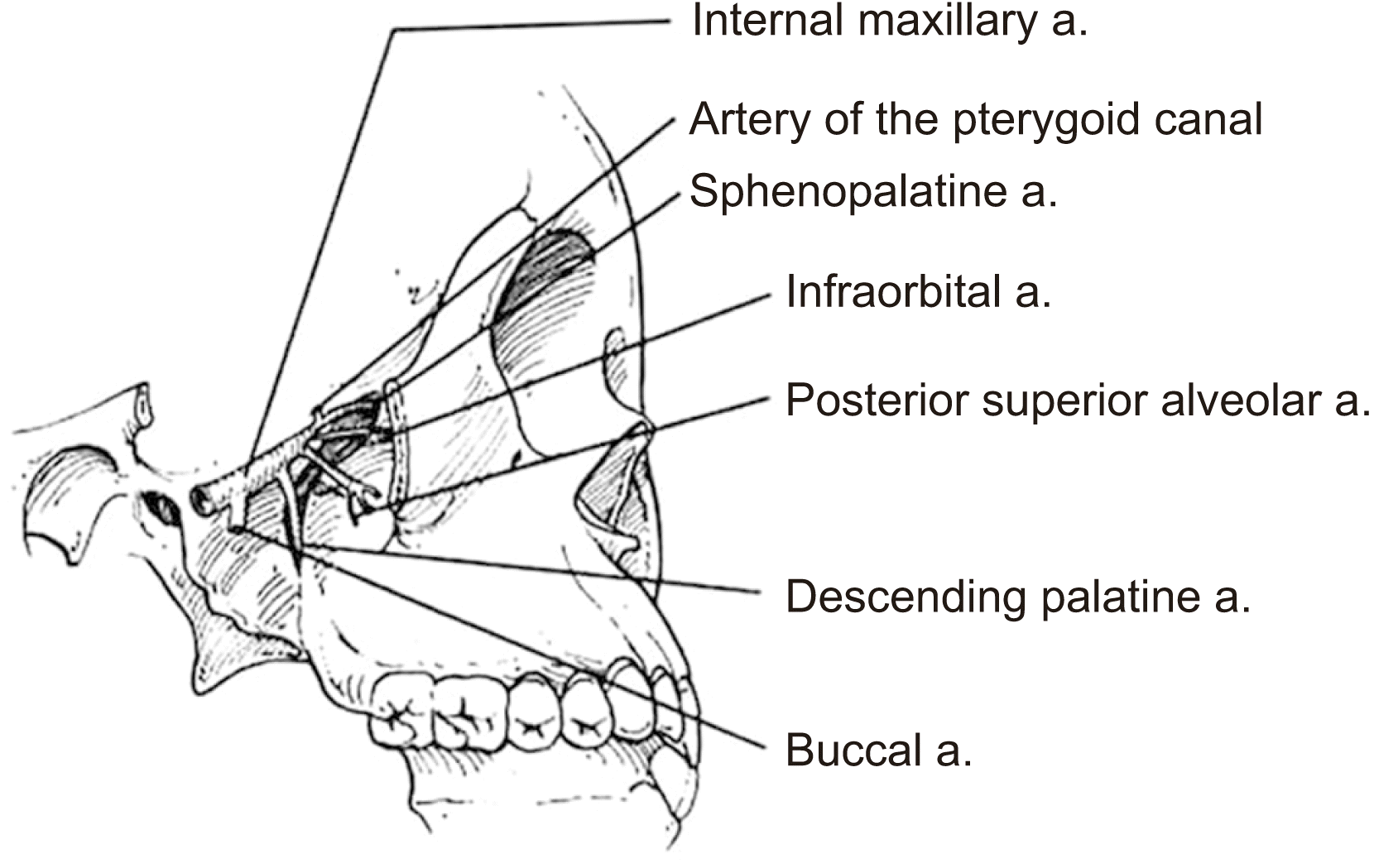
Fig. 2
Anterior nasal packing for epistaxis. 1Lubricated or antibiotic-soaked gauze was inserted into the anterior nasal cavity. 2Nasopharynx.
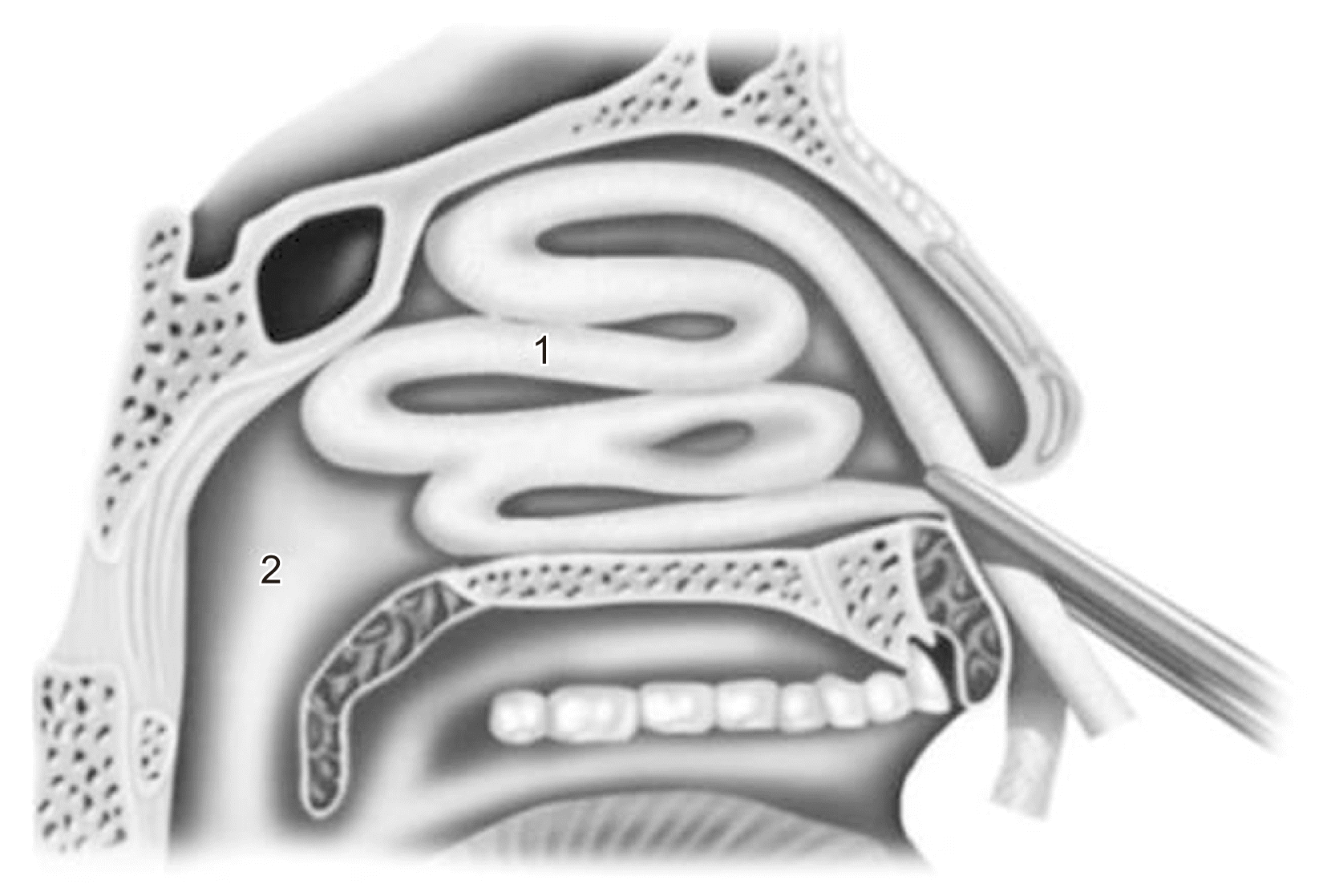
Fig. 3
Posterior nasal packing for posterior epistaxis using a 1Foley catheter balloon. 2Soft palate.
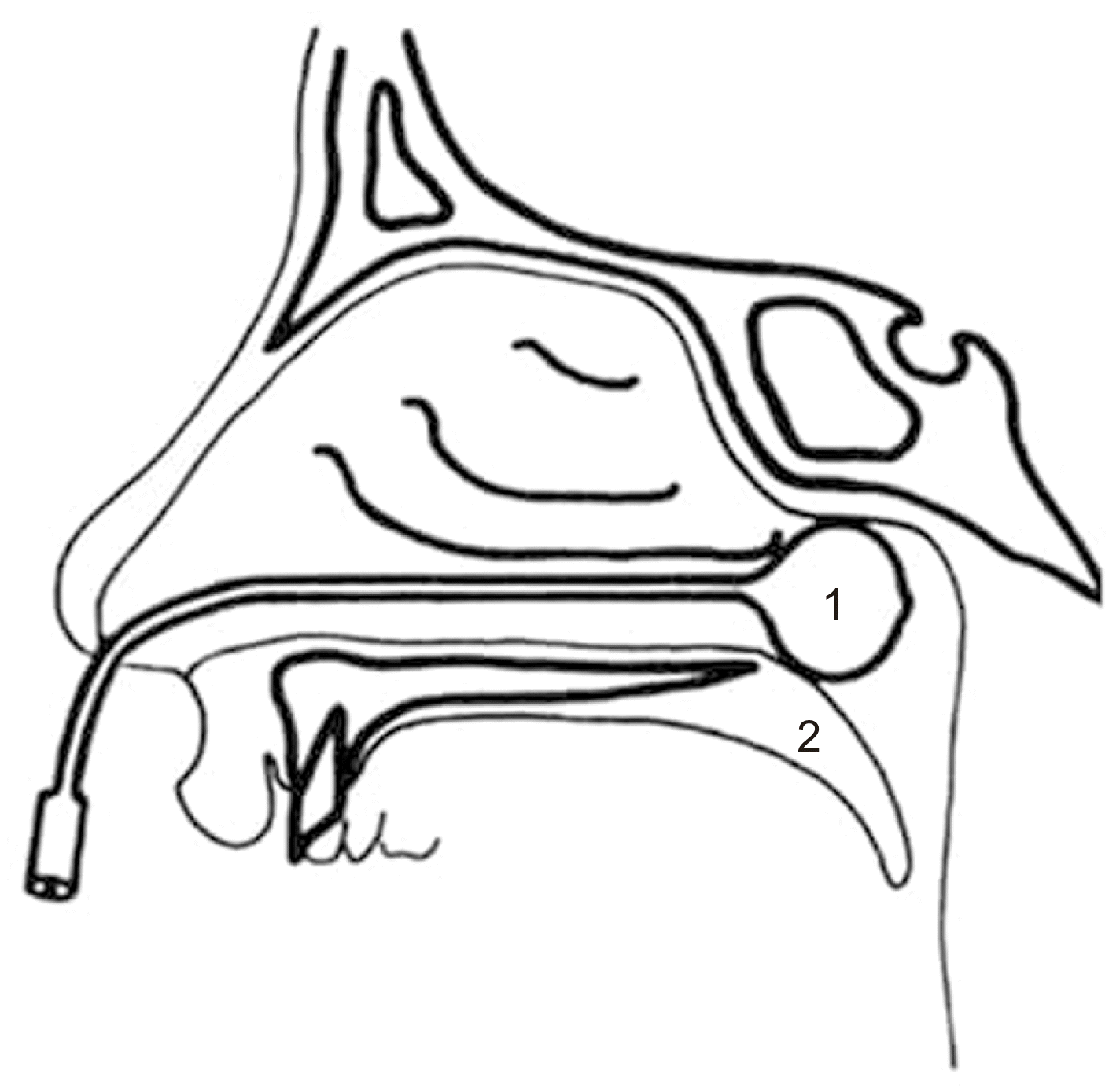
Table 1
Studies reporting epistaxis as complication after dental-implant surgery
| Study | Article type | Technique | Total No. of patients | No. of patients with epistaxis |
|---|---|---|---|---|
| Nooh9 | Retrospective study | Sinus lift with membrane perforation | 56 | 7 |
| Kim et al.10 | Retrospective study | Sinus membrane penetration | 39 | 3 |
| Shihab11 | Retrospective study | Sinus lift with membrane perforation | 35 | 3 |
| Bae et al.12 | Retrospective study |
Reamer-mediated Osteotome-mediated |
40 45 |
2 3 |
| Hong and Mun8 | Case report | Dental implantation | 1 | 1 |
Table 2
Studies reporting epistaxis as a complication after Le Fort osteotomy
| Study | Article type | No. of patients | Age group (yr) | Surgical approach | No. of patients with epistaxis | Postoperative onset of epistaxis |
|---|---|---|---|---|---|---|
| Williams et al.15 | Retrospective study | 120 | 22-39 | SARPE | 7 | 1 hour to 7 days |
| Lanigan et al.13 | Questionnaire | NM | 18-23 | Le Fort I osteotomy | 5 | 18 hours to 10 days |
| de Mol van Otterloo et al.16 | Retrospective study | 410 | NM | Le Fort I osteotomy, SARPE | 3 | Several days |
| Eshghpour et al.17 | Prospective study | 114 | 18-30 | Le Fort I osteotomy | 2 | First 24 hours |
| Avelar et al.18 | Case report | 1 | 20 | Le Fort I osteotomy | 1 | 9 weeks |
| Park et al.19 | Case report | 1 | 30 | Le Fort I osteotomy | 1 | 3 weeks |
| Mehra et al.14 | Case report | 1 | 25 | SARPE | 1 | 11 days |
| Solomons and Blumgart2 | Case report | 1 | 20 | Le Fort I osteotomy | 1 | One month |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download