INTRODUCTION
METHODS
Cell culture and transfection
Electrophysiology
Data analysis
RESULTS
Inhibition of Kv1.5 by rosiglitazone
 | Fig. 1Concentration dependence of rosiglitazone-induced inhibition of Kv1.5 currents.(A) Kv1.5 currents were elicited with +50 mV depolarization (250 ms) from a holding potential of –80 mV, every 10 sec. Currents recorded in the absence and presence of 3, 10, 30, and 100 µM rosiglitazone were superimposed. The dotted line represents zero current. (B) Dose response curve of rosiglitazone-mediated reduction in Kv1.5 current. The amplitudes of Kv1.5 current were measured at the end (closed circle) and peak (open circle) of the depolarizing pulses at various concentrations of rosiglitazone. The data of % inhibition (I [%] = [1 – Irosiglitazone/Icontrol] × 100) were fitted with the Hill equation (solid lines). (C) Time course of Kv1.5 inhibition by rosiglitazone. The current amplitudes were measured at the end of a 250-ms depolarizing pulse and normalized to the baseline amplitude. Data are expressed as mean ± SEM.
|
Voltage-dependence of rosiglitazone-mediated inhibition of Kv1.5
 | Fig. 2Voltage dependence of rosiglitazone-induced inhibition of Kv1.5 currents.The Kv1.5 currents were produced by applying 250-ms depolarizing pulses between –60 and +40 mV in 10-mV increments every 10 sec from a holding potential of –80 mV under control conditions (A), and after the addition of 20 µM rosiglitazone (B). The dotted lines in (A) and (B) represent zero current. (C) The amplitudes of Kv1.5 currents were measured at the end of test pulses and plotted against membrane potentials, in control (○) and 20 µM rosiglitazone (●). (D) The activation curve of control Kv1.5 current was constructed from tail current amplitudes at –40 mV after 250-ms depolarizing pulses between –60 and +40 mV in 10-mV. Only the Boltzmann fitting curve is shown (dotted line, normalized current y-axis for activation curve). The percent inhibition of Kv1.5 current by rosiglitazone was plotted (●) for comparison with the activation profile. In the voltage range between –20 and 0 mV, the percent inhibition increased steeply suggesting an open channel blockade. The solid line was drawn from a linear curve fitting the relative current data between 0 and +40 mV. Data are expressed as mean ± SEM.
|
Decay kinetics of Kv1.5 currents in rosiglitazone
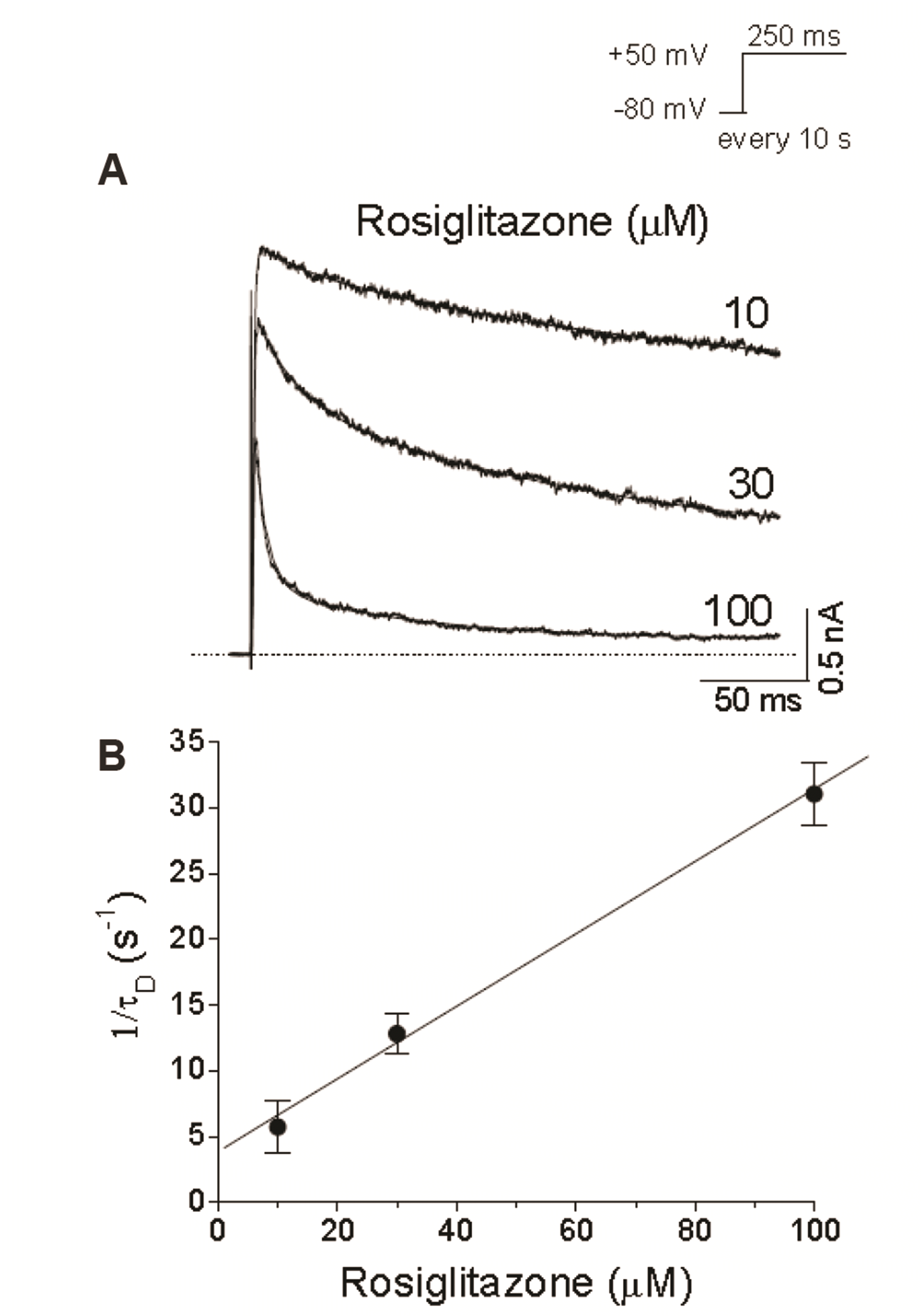 | Fig. 3Concentration-dependent kinetics of Kv1.5 inhibition caused by rosiglitazone.(A) Kv1.5 currents were elicited with +50 mV pulses (250 ms) every 10 sec in the presence of rosiglitazone (10, 30, and 100 µM). The dotted line represents zero current. (B) A drug-induced time constant (τD) was obtained from a single exponential fitting to the decaying phase of Kv1.5 current. The inverse of τD obtained at +50 mV was plotted against rosiglitazone concentrations. The solid line represents linear fit of the data. A binding rate constant (k+1) and an unbinding rate constant (k–1) were obtained from the slope and intercept values of the fitted line, 1/τD = k+1[D] + k–1. Data are expressed as mean ± SEM.
|
Deactivation kinetics
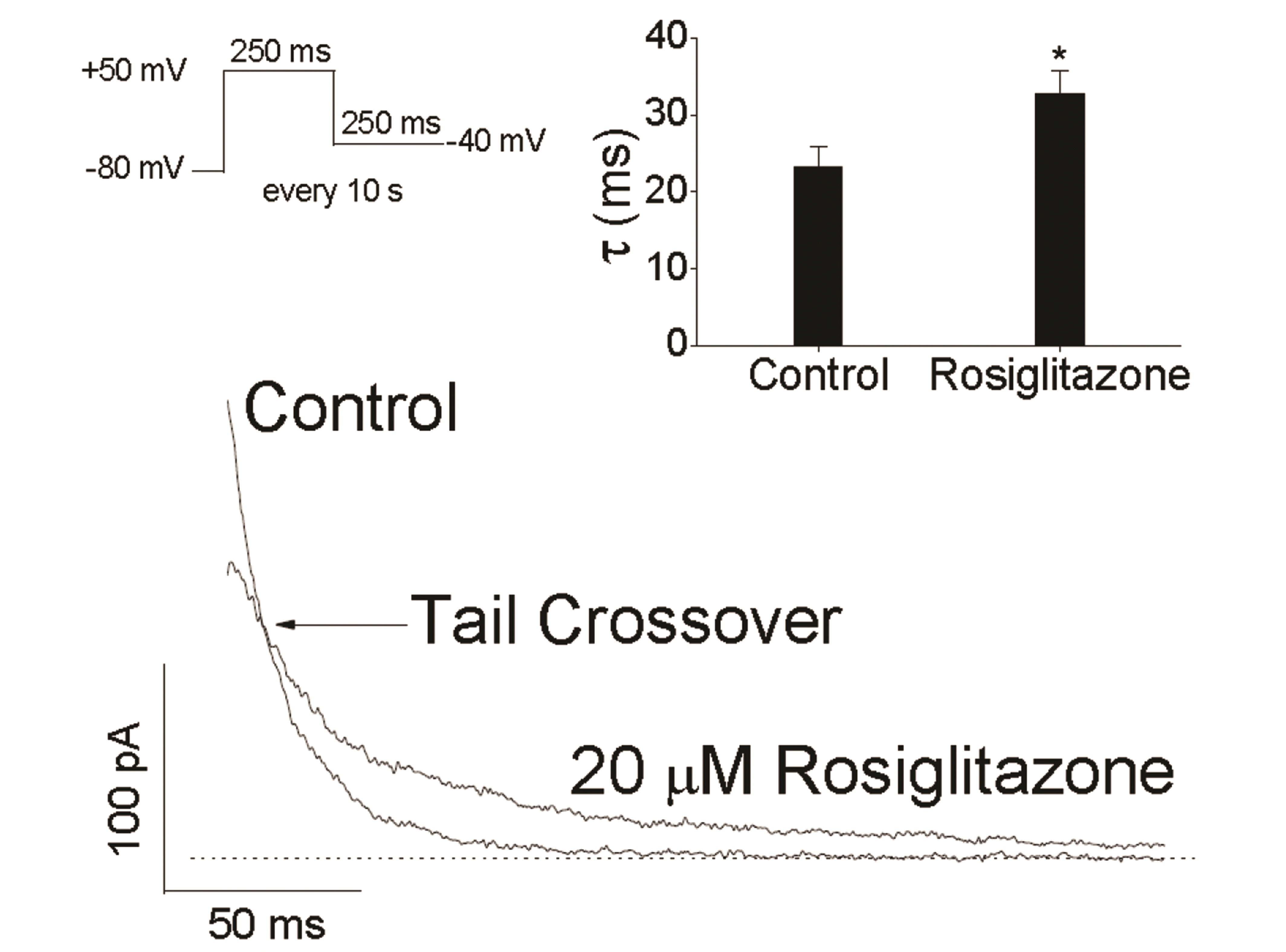 | Fig. 4Effects of rosiglitazone on the deactivation kinetics of Kv1.5 currents.Tail currents were induced with the repolarizing pulse of –40 mV after a 250-ms depolarizing pulse of +50 mV, in the absence and presence of 20 µM rosiglitazone. Superimposition of two tail currents revealed tail crossover (arrow). The dotted line represents zero current. Inset, mean time constants of the tail current decay in the presence of rosiglitazone was significantly larger than that in control condition (Student’s t-test, n = 4; *p < 0.05).
|
Use-dependent inhibition of Kv1.5 by rosiglitazone
 | Fig. 5Use-dependence of rosiglitazone-mediated inhibition of Kv1.5.(A) Superimposed Kv1.5 current traces elicited successively with depolarizing pulses (+50 mV, 125 ms) at 1 or 2 Hz. The dotted lines represent zero current. Dashed lines are for a comparison of the peak amplitudes of the first currents in both stimuli sets. (B) Plot of normalized amplitude of peak current 1 (circles) and 2 Hz (triangles), in control (open symbols) and in 20 µM rosiglitazone (closed symbols), against the elapsed time axis. The peak amplitudes were normalized to the peak amplitude of the first current in each condition. (C) With the data in (B), the current amplitude in rosiglitazone was normalized to the control amplitude at each elapsed time. Data are expressed as mean ± SEM.
|
Effects of rosiglitazone on the kinetics of Kv1.5 recovery from inactivation
 | Fig. 6Effects of rosiglitazone on the kinetics of Kv1.5 recovery from steady-state inactivation.(A) The degree of recovery was assayed with the following two-pulse protocol: the first prepulse (+40 mV, 250 ms) was followed by a second identical pulse with a varying interpulse interval ranging from 5 to 10,000 ms. The pair of pulses was applied at an interval of 30 sec. (B) The peak amplitude of the second current in the pair (■ in A) was normalized to the peak of the first current (□ in A), and plotted against interpulse intervals. (C) The range of y-axis values in (B) were expanded to the range from 0 to 1 by normalizing the minimum values in (B) (at 5 ms interval) to 0. This re-normalization revealed faster recovery from inactivation in rosiglitazone than in control condition. The curves in (B and C) were obtained from a double exponential fitting, from which two recovery time constants were obtained. (D) Summarized data of the fast and slow time constants obtained from the double exponential fittings (n = 4; *p < 0.05 vs. data under control conditions). Data are expressed as means ± SEM.
|




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download