1. Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. 1992; Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 258:1946–1949. DOI:
10.1126/science.1470919. PMID:
1470919.

2. Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. 1995; 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 215:89–97. DOI:
10.1006/bbrc.1995.2437. PMID:
7575630.

3. Cravatt BF, Prospero-Garcia O, Siuzdak G, Gilula NB, Henriksen SJ, Boger DL, Lerner RA. 1995; Chemical characterization of a family of brain lipids that induce sleep. Science. 268:1506–1509. DOI:
10.1126/science.7770779. PMID:
7770779.

6. Zou S, Kumar U. 2018; Cannabinoid receptors and the endocannabinoid system: signaling and function in the central nervous system. Int J Mol Sci. 19:833. DOI:
10.3390/ijms19030833. PMID:
29533978. PMCID:
PMC5877694.

8. Aghazadeh Tabrizi M, Baraldi PG, Borea PA, Varani K. 2016; Medicinal chemistry, pharmacology, and potential therapeutic benefits of cannabinoid CB
2 receptor agonists. Chem Rev. 116:519–560. DOI:
10.1021/acs.chemrev.5b00411. PMID:
26741146.

9. Rom S, Zuluaga-Ramirez V, Dykstra H, Reichenbach NL, Pacher P, Persidsky Y. 2013; Selective activation of cannabinoid receptor 2 in leukocytes suppresses their engagement of the brain endothelium and protects the blood-brain barrier. Am J Pathol. 183:1548–1558. DOI:
10.1016/j.ajpath.2013.07.033. PMID:
24055259. PMCID:
PMC3814716.

10. Page RL 2nd, Allen LA, Kloner RA, Carriker CR, Martel C, Morris AA, Piano MR, Rana JS, Saucedo JF. American Heart Association Clinical Pharmacology Committee and Heart Failure and Transplantation Committee of the Council on Clinical Cardiology. Council on Basic Cardiovascular Sciences. Council on Cardiovascular and Stroke Nursing. Council on Epidemiology and Prevention. Council on Lifestyle and Cardiometabolic Health. Council on Quality of Care and Outcomes Research. 2020; Medical marijuana, recreational cannabis, and cardiovascular health: a scientific statement from the American Heart Association. Circulation. 142:e131–e152. DOI:
10.1161/CIR.0000000000000883. PMID:
32752884.

11. López-Ortíz M, Herrera-Solís A, Luviano-Jardón A, Reyes-Prieto N, Castillo I, Monsalvo I, Demare P, Méndez-Díaz M, Regla I, Prospéro-García O. 2010; Chemoenzymatic synthesis and cannabinoid activity of a new diazabicyclic amide of phenylacetylricinoleic acid. Bioorg Med Chem Lett. 20:3231–3234. DOI:
10.1016/j.bmcl.2010.04.074. PMID:
20457524.

12. Vidrio H, Medina M, González-Romo P, Lorenzana-Jiménez M, Díaz-Arista P, Baeza A. 2003; Semicarbazide-sensitive amine oxidase substrates potentiate hydralazine hypotension: possible role of hydrogen peroxide. J Pharmacol Exp Ther. 307:497–504. DOI:
10.1124/jpet.103.055350. PMID:
12970383.

13. Maggi CA, Meli A. 1986; Suitability of urethane anesthesia for physiopharmacological investigations in various systems. Part 2: cardiovascular system. Experientia. 42:292–297. DOI:
10.1007/BF01942510. PMID:
3007197.

14. Furchgott RF, Zawadzki JV. 1980; The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 288:373–376. DOI:
10.1038/288373a0. PMID:
6253831.

15. Sudhahar V, Shaw S, Imig JD. 2009; Mechanisms involved in oleamide-induced vasorelaxation in rat mesenteric resistance arteries. Eur J Pharmacol. 607:143–150. DOI:
10.1016/j.ejphar.2009.02.002. PMID:
19326479. PMCID:
PMC2664517.

16. Quiñonez-Bastidas GN, Palomino-Hernández O, López-Ortíz M, Rocha-González HI, González-Anduaga GM, Regla I, Navarrete A. 2020; Antiallodynic effect of PhAR-DBH-Me involves cannabinoid and TRPV1 receptors. Pharmacol Res Perspect. 8:e00663. DOI:
10.1002/prp2.663. PMID:
32965798. PMCID:
PMC7510332.

17. Ligresti A, De Petrocellis L, Di Marzo V. 2016; From phytocannabinoids to cannabinoid receptors and endocannabinoids: pleiotropic physiological and pathological roles through complex pharmacology. Physiol Rev. 96:1593–1659. DOI:
10.1152/physrev.00002.2016. PMID:
27630175.

19. Hebert-Chatelain E, Desprez T, Serrat R, Bellocchio L, Soria-Gomez E, Busquets-Garcia A, Pagano Zottola AC, Delamarre A, Cannich A, Vincent P, Varilh M, Robin LM, Terral G, García-Fernández MD, Colavita M, Mazier W, Drago F, Puente N, Reguero L, Elezgarai I, et al. 2016; A cannabinoid link between mitochondria and memory. Nature. 539:555–559. DOI:
10.1038/nature20127. PMID:
27828947.

20. Mendizabal-Zubiaga J, Melser S, Bénard G, Ramos A, Reguero L, Arrabal S, Elezgarai I, Gerrikagoitia I, Suarez J, Rodríguez De Fonseca F, Puente N, Marsicano G, Grandes P. 2016; Cannabinoid CB
1 receptors are localized in striated muscle mitochondria and regulate mitochondrial respiration. Front Physiol. 7:476. DOI:
10.3389/fphys.2016.00476. PMID:
27826249. PMCID:
PMC5078489.
21. Bonz A, Laser M, Küllmer S, Kniesch S, Babin-Ebell J, Popp V, Ertl G, Wagner JA. 2003; Cannabinoids acting on CB
1 receptors decrease contractile performance in human atrial muscle. J Cardiovasc Pharmacol. 41:657–664. DOI:
10.1097/00005344-200304000-00020. PMID:
12658069.

22. Chen XP, Yang W, Fan Y, Luo JS, Hong K, Wang Z, Yan JF, Chen X, Lu JX, Benovic JL, Zhou NM. 2010; Structural determinants in the second intracellular loop of the human cannabinoid CB
1 receptor mediate selective coupling to G
S and G
i. Br J Pharmacol. 161:1817–1834. DOI:
10.1111/j.1476-5381.2010.01006.x. PMID:
20735408. PMCID:
PMC3010585.

24. Lozovaya N, Min R, Tsintsadze V, Burnashev N. 2009; Dual modulation of CNS voltage-gated calcium channels by cannabinoids: focus on CB
1 receptor-independent effects. Cell Calcium. 46:154–162. DOI:
10.1016/j.ceca.2009.07.007. PMID:
19682741.

25. Wheal AJ, Bennett T, Randall MD, Gardiner SM. 2007; Cardiovascular effects of cannabinoids in conscious spontaneously hypertensive rats. Br J Pharmacol. 152:717–724. DOI:
10.1038/sj.bjp.0707410. PMID:
17700721. PMCID:
PMC2190006.

27. Lépicier P, Bouchard JF, Lagneux C, Lamontagne D. 2003; Endocannabinoids protect the rat isolated heart against ischaemia. Br J Pharmacol. 139:805–815. DOI:
10.1038/sj.bjp.0705313. PMID:
12813004. PMCID:
PMC1573907.

28. Underdown NJ, Hiley CR, Ford WR. 2005; Anandamide reduces infarct size in rat isolated hearts subjected to ischaemia-reperfusion by a novel cannabinoid mechanism. Br J Pharmacol. 146:809–816. DOI:
10.1038/sj.bjp.0706391. PMID:
16158067. PMCID:
PMC1751211.

29. Al Kury LT, Voitychuk OI, Yang KH, Thayyullathil FT, Doroshenko P, Ramez AM, Shuba YM, Galadari S, Howarth FC, Oz M. 2014; Effects of the endogenous cannabinoid anandamide on voltage-dependent sodium and calcium channels in rat ventricular myocytes. Br J Pharmacol. 171:3485–3498. DOI:
10.1111/bph.12734. PMID:
24758718. PMCID:
PMC4105935.

30. Hoi PM, Hiley CR. 2006; Vasorelaxant effects of oleamide in rat small mesenteric artery indicate action at a novel cannabinoid receptor. Br J Pharmacol. 147:560–568. DOI:
10.1038/sj.bjp.0706643. PMID:
16415907. PMCID:
PMC1616976.

31. Kozłowska H, Baranowska M, Schlicker E, Kozłowski M, Laudański J, Malinowska B. 2007; Identification of the vasodilatory endothelial cannabinoid receptor in the human pulmonary artery. J Hypertens. 25:2240–2248. DOI:
10.1097/HJH.0b013e3282ef7a0a. PMID:
17921818.

32. Lépicier P, Lagneux C, Sirois MG, Lamontagne D. 2007; Endothelial CB
1-receptors limit infarct size through NO formation in rat isolated hearts. Life Sci. 81:1373–1380. DOI:
10.1016/j.lfs.2007.08.042. PMID:
17931662.

33. Silva BR, Pernomian L, De Paula TD, Grando MD, Bendhack LM. 2017; Endothelial nitric oxide synthase and cyclooxygenase are activated by hydrogen peroxide in renal hypertensive rat aorta. Eur J Pharmacol. 814:87–94. DOI:
10.1016/j.ejphar.2017.07.047. PMID:
28780058.

34. Herradón E, Martín MI, López-Miranda V. 2007; Characterization of the vasorelaxant mechanisms of the endocannabinoid anandamide in rat aorta. Br J Pharmacol. 152:699–708. DOI:
10.1038/sj.bjp.0707404. PMID:
17704831. PMCID:
PMC2190007.

35. Romano MR, Lograno MD. 2006; Cannabinoid agonists induce relaxation in the bovine ophthalmic artery: evidences for CB
1 receptors, nitric oxide and potassium channels. Br J Pharmacol. 147:917–925. DOI:
10.1038/sj.bjp.0706687. PMID:
16474412. PMCID:
PMC1760716.

36. Hoyer FF, Steinmetz M, Zimmer S, Becker A, Lütjohann D, Buchalla R, Zimmer A, Nickenig G. 2011; Atheroprotection via cannabinoid receptor-2 is mediated by circulating and vascular cells in vivo. J Mol Cell Cardiol. 51:1007–1014. DOI:
10.1016/j.yjmcc.2011.08.008. PMID:
21884703.

37. González C, Herradón E, Abalo R, Vera G, Pérez-Nievas BG, Leza JC, Martín MI, López-Miranda V. 2011; Cannabinoid/agonist WIN 55,212-2 reduces cardiac ischaemia-reperfusion injury in Zucker diabetic fatty rats: role of CB
2 receptors and iNOS/eNOS. Diabetes Metab Res Rev. 27:331–340. DOI:
10.1002/dmrr.1176. PMID:
21309057.

38. Járai Z, Wagner JA, Varga K, Lake KD, Compton DR, Martin BR, Zimmer AM, Bonner TI, Buckley NE, Mezey E, Razdan RK, Zimmer A, Kunos G. 1999; Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB
1 or CB
2 receptors. Proc Natl Acad Sci U S A. 96:14136–14141. DOI:
10.1073/pnas.96.24.14136. PMID:
10570211. PMCID:
PMC24203.

40. Hecker M, Bara AT, Bauersachs J, Busse R. 1994; Characterization of endothelium-derived hyperpolarizing factor as a cytochrome P450-derived arachidonic acid metabolite in mammals. J Physiol. 481(Pt 2):407–414. DOI:
10.1113/jphysiol.1994.sp020449. PMID:
7738833. PMCID:
PMC1155939.

41. Wheal AJ, Alexander SP, Randall MD. 2010; Vasorelaxation to N-oleoylethanolamine in rat isolated arteries: mechanisms of action and modulation via cyclooxygenase activity. Br J Pharmacol. 160:701–711. DOI:
10.1111/j.1476-5381.2010.00770.x. PMID:
20590573. PMCID:
PMC2931569.

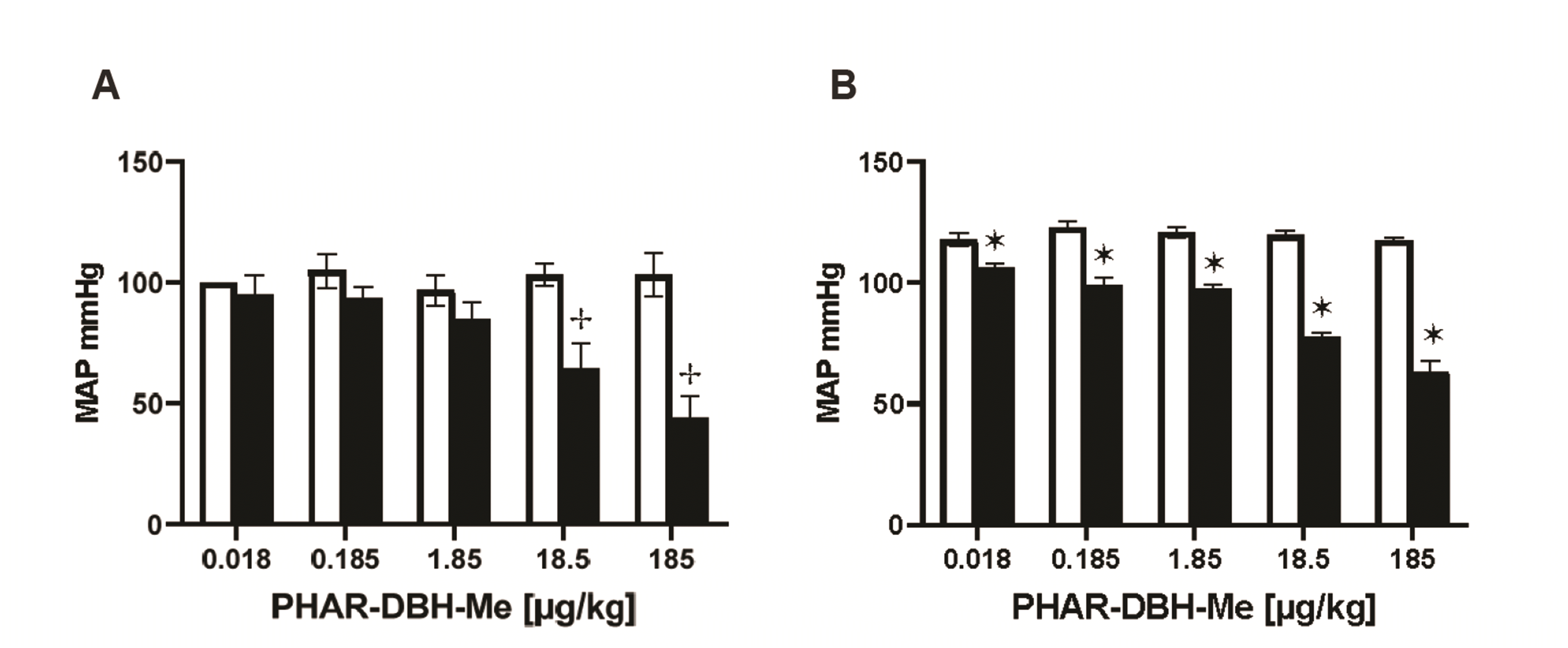
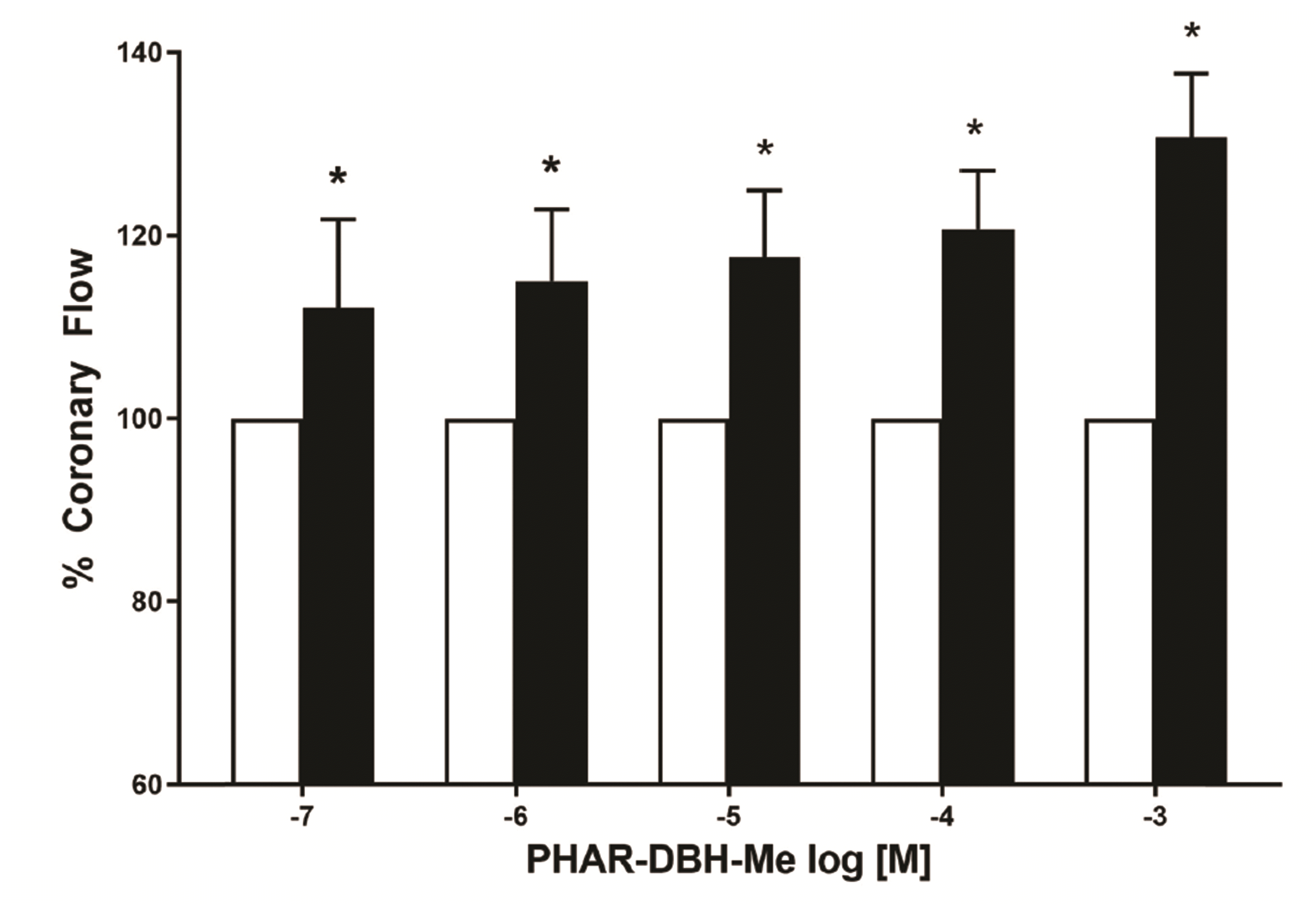
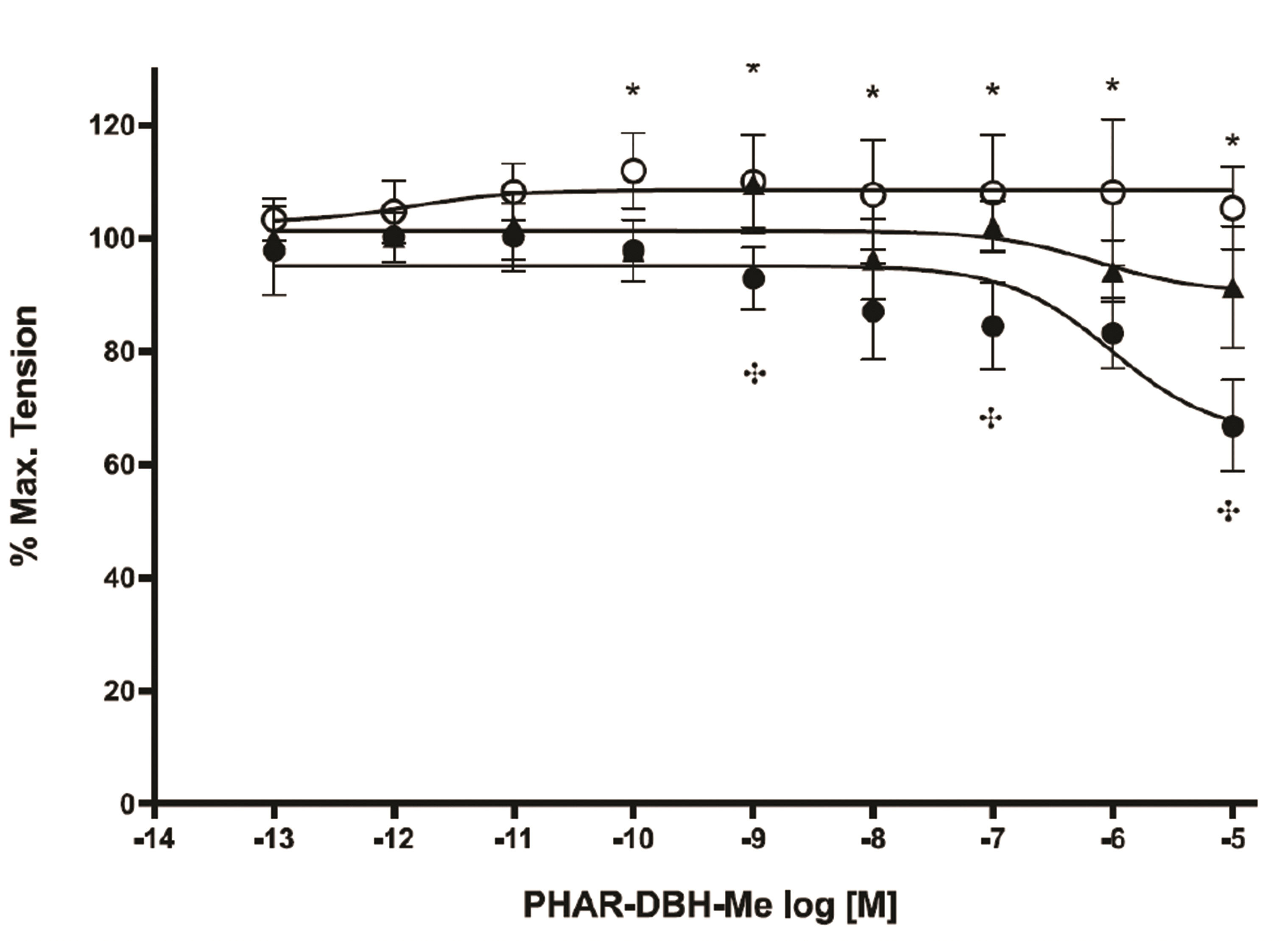
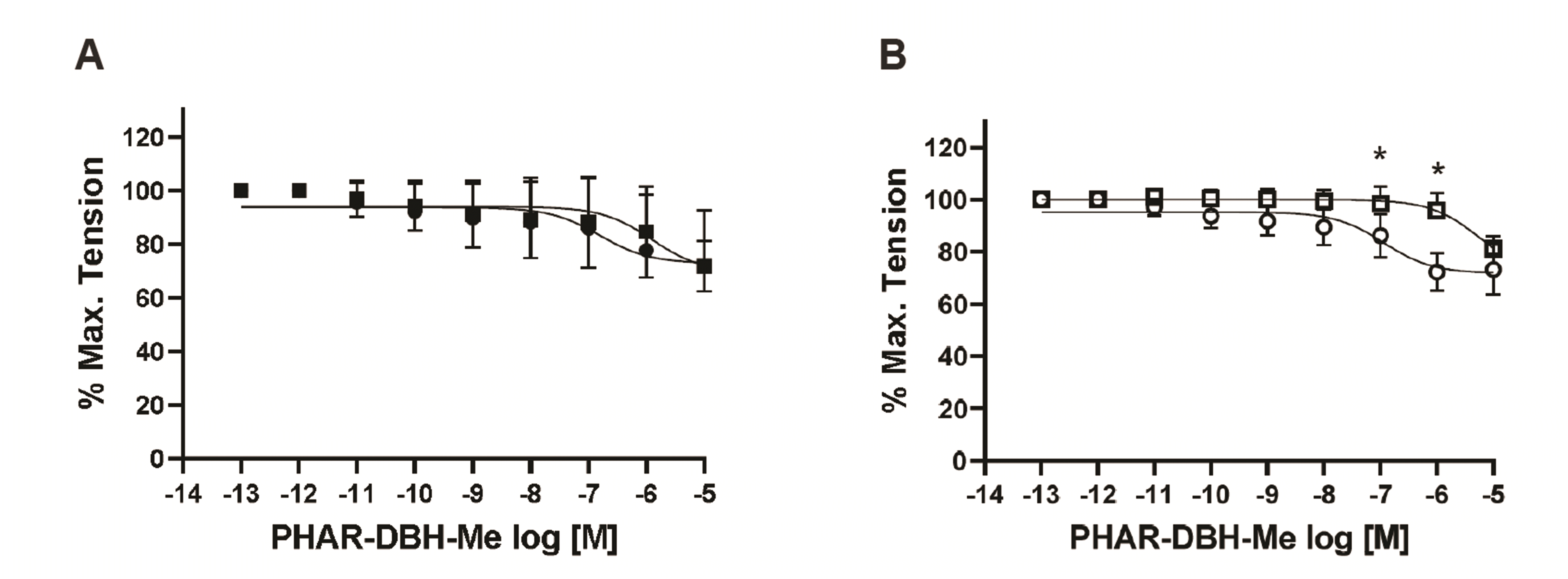




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download