Abstract
Neurolymphomatosis (NL) is defined as the involvement of the peripheral nervous system in lymphocytic invasion. It is a very rare form of lymphoma that may occur as an initial presentation or recurrence. It affects various peripheral nervous structures and can therefore mimic disc-related nerve root pathology or compressive mononeuropathy. NL often occurs in malignant B-cell non-Hodgkin lymphomas. Notwithstanding its aggressiveness or intractability, NL should be discriminated from other neurologic complications of lymphoma. Herein, we present a case of primary NL as the initial presentation of diffuse large B-cell lymphoma (DLBCL) of the sciatic nerve. The patient presented with weakness and pain in his left leg but had no obvious lesion explaining the neurologic deficit on initial lumbosacral and knee magnetic resonance imaging (MRI). NL of the left sciatic nerve at the greater sciatic foramen was diagnosed based on subsequent hip MRI, electrodiagnostic test, positron emission tomography/computed tomography, and nerve biopsy findings. Leg weakness slightly improved after chemotherapy and radiotherapy. We report a case wherein NL, a rare cause of leg weakness, manifested as the initial presentation of primary DLBCL involving the sciatic nerve at the greater sciatic foramen.
Neurolymphomatosis (NL), defined as lymphocytic invasion of the peripheral nervous system in a hematologic malignancy setting, is a rare form of lymphoma that may occur as an initial presentation or recurrence [1]. Involvement of the peripheral nerves, spinal nerve roots, nerve plexus, cranial nerves, and multiple sites has been reported [2]. Since its characteristic symptoms vary according to the site of involvement, physicians might fail to obtain an accurate diagnosis. NL should be discriminated from other neurological complications of lymphoma, such as compressive neuropathy due to tumor mass, paraneoplastic neuropathy, toxic neuropathy after chemotherapy, and neuropathy following radiotherapy [2]. Although NL often results from systemic dissemination of lymphoma or direct extension of a contiguous lesion into the nerve (secondary NL), it can also occur as isolated peripheral nervous system involvement with malignant lymphocytes at initial presentation (primary NL) [3,4]. As primary peripheral NL is extremely rare, only a few cases of primary lymphoma of the sciatic nerve have been reported [3,5-7]. Here, we present a rare case of primary NL as the initial presentation of diffuse large B-cell lymphoma (DLBCL) of the sciatic nerve with a brief review of the literature.
A 57-year-old male presented to the rehabilitation department with weakness and pain in his left leg for 2 months. The manual muscle test grades of his left lower extremity were as follows: knee flexion and ankle plantar flexion, grade 3 and ankle dorsiflexion and great toe extension, grade 0. Left ankle deep tendon reflex was absent. He had hypesthesia and allodynia in the left L5 and S1 dermatomes. Lumbosacral and knee magnetic resonance imaging (MRI) showed no neural compressive lesions that could induce neurologic deficits.
Electrodiagnostic tests performed to detect neurologic compromise revealed left lumbosacral plexopathy with denervation potentials in the left inferior gluteal nerve. Hip MRI with contrast showed a homogeneously enhancing, 6.3×2.9×2.8 cm-sized, fusiform, enlarged mass at the left greater sciatic foramen with diffuse hypertrophy from the left L5 and S1 nerve roots to the sciatic nerve (Fig. 1A), suggestive of lymphoma infiltration or neurogenic tumors such as perineurioma and neurofibroma.
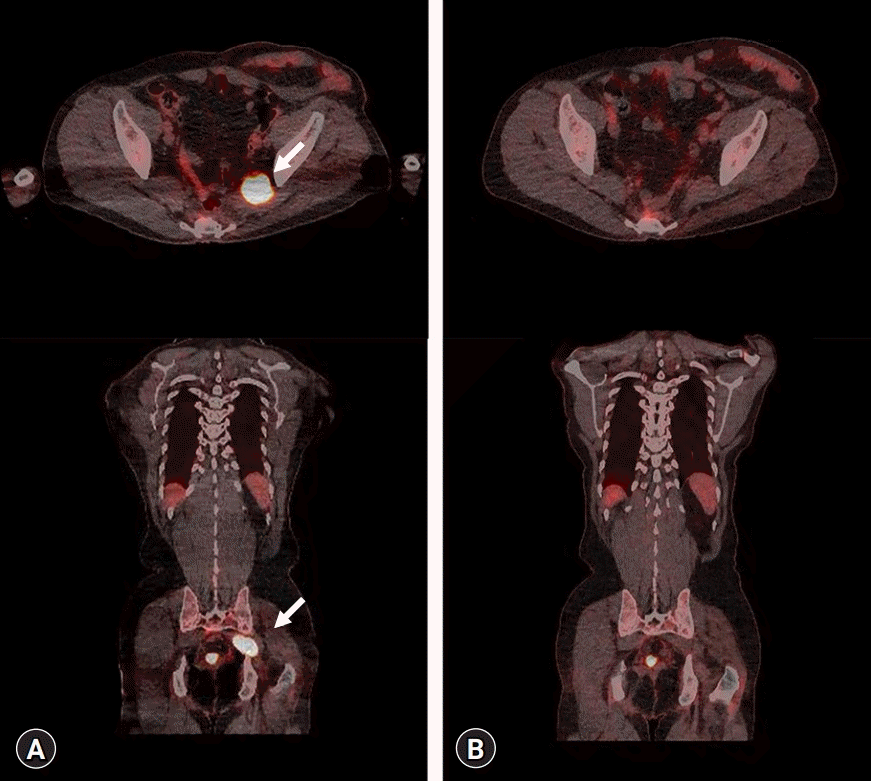
Ultrasound-guided needle aspiration of the left sciatic nerve was performed for histopathologic confirmation. Considering the characteristics of the sciatic nerve, which is a major nerve that includes both motor and sensory components, needle aspiration was performed by targeting the soft tissue adjacent to the sciatic nerve to minimize nerve damage. Immunohistochemistry revealed tumor cells with infiltration of lymphocytes in the sciatic nerve (Fig. 2A). Lymphoma cells were strongly positive for CD20 (Fig. 2B). Consequently, the patient was diagnosed with a non-germinal center B-cell phenotype of DLBCL. Fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT), bone marrow biopsy, and cervical, thoracic, and abdominal CT were performed. FDG PET/CT showed diffuse increased uptake of FDG in the left sciatic nerve and a few hypermetabolic foci in the left presacral area and pelvic cavity (Fig. 3A). The bone marrow biopsy showed positive expression of CD79, PAX-5, and CD3 and negative immunoreactivity for CD20 (data not shown). The patient was scheduled to undergo chemotherapy based on the biopsy results, and prednisolone was first administered to reduce inflammation and swelling of the lesion site. No symptom improvement was observed after daily administration of prednisolone (100 mg) for 5 days. Subsequently, the patient received six cycles of chemotherapy (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone [R-CHOP]).
On posttreatment hip MRI, the size of the enhancing mass at the sciatic foramen was markedly decreased (Fig. 1B). FDG PET/CT showed complete metabolic resolution of the mass at the left sacral foramen and partial metabolic resolution of the hypermetabolic foci in the left presacral area and pelvic cavity (Fig. 3B). Systemic involvement or recurrence was not observed. A follow-up electrodiagnostic study revealed left lumbosacral plexopathy with some axonal regeneration compared to the initial study. The patient showed slight motor improvement, equivalent to one grade on manual muscle testing, in all previously weakened muscles. The patient recently underwent adjuvant radiotherapy and is being followed up at our hospital’s departments of internal medicine and rehabilitation medicine. He is undergoing rehabilitation to maintain leg muscle mass and prevent tightness at the ankle joints.
NL is an uncommon form of initial presentation or recurrence of lymphoma. It was described by Lhermitte and Trelles [8] in 1934 as a localized invasion of malignant lymphoid cells into peripheral nerves, roots, and cranial nerves. The nerve structures involved are peripheral nerves (60% of cases), spinal nerve roots (48% of cases), nerve plexus (40% of cases), cranial nerves (46% of cases), and multiple sites (58% of cases) [9]. NL often occurs in malignant B-cell non-Hodgkin lymphoma (NHL), which is aggressive, and systemic involvement is common [9]. DLBCL is the most common presentation of NHL, with approximately 40% of cases representing extranodal disease [5]. NL is a unique expression of extranodal NHL that accounts for 0.85% to 2.9% of newly diagnosed cases with neurological compromise [1].
Because of its rarity, the exact incidence of NL has not yet been clarified. A study conducted by the International Primary Central Nervous System Lymphoma Collaborative Group reported that 26% to 29.5% of all NL cases represented an initial presentation of systemic lymphoma, with a variable incidence of peripheral nerve involvement (20%–66%) [9]. Peripheral nerve involvement in NL is either due to systemic dissemination of lymphoma or direct extension of a contiguous lesion into the nerve [3]. Although less common, primary NL with isolated peripheral nerve involvement at initial presentation has also been reported.
Primary NL without systemic involvement is extremely rare and tends to have a predilection for the sciatic nerve [6,10,11]. The pathogenesis of this preferential involvement of the sciatic nerve has been debated. Baehring et al. [12] postulated that specific adhesion receptors on lymphoma cells that are analogous to normal lymphoid cells might lead to the involvement of specific tissues. Quiñones-Hinojosa et al. [13] suggested that the original lymphoma might be derived from B cells from or around the sciatic nerve.
NL presents with pure demyelinating or mixed axonal and demyelinating neuropathy, mononeuropathy, or symmetrical neuropathy [14]. Based on clinical features, NL has been classified into four types; (1) painful involvement of nerves or roots, (2) painless involvement of peripheral nerves, (3) painful or painless involvement of a single peripheral nerve, and (4) painful or painless cranial neuropathy [1]. Painful involvement of nerves or roots is the most common type. Consistent with this finding, our patient had pain in the L5 dermatome and ankle weakness corresponding to the L5 and S1 myotomes. As mentioned above, these symptoms are similar to those seen in L5 or S1 radiculopathy, lumbosacral plexopathy, and common peroneal neuropathy. Furthermore, distal lower limb weakness may be a clinical feature of motor neuron disease, distal myopathy, and polyneuropathy. These differential diagnoses must be considered in the clinical diagnostic approaches. Differentiating between NL and schwannoma on CT or MRI is sometimes challenging [6].
Histopathologic findings of peripheral nerve invasion by the tumor on nerve biopsy may be the most reliable method for a confirmed diagnosis of NL. However, it is not considered the diagnostic modality of choice for NL because of its invasive nature. There are several alternative imaging modalities, including MRI and FDG PET/CT, which can be used to diagnose NL. Previous studies have shown that FDG PET/CT is the most potent imaging tool for patients with suspected NL and has higher sensitivity than gadolinium-enhanced MRI [9,15]. Jeong et al. [4] reported that the sensitivities of FDG PET/CT and MRI for detecting NL were 100% and 78%, respectively. In this study, the patient underwent both MRI and FDG PET/CT before and after treatment. After treatment, the size of the enhanced mass was significantly decreased on MRI, and hypermetabolic foci showed partial remission on FDG PET/CT. Nevertheless, his neurologic weakness only improved slightly. This mismatch between the neurologic and metabolic responses could be due to a long time interval between symptom onset and treatment initiation, which could have led to irreversible axonal damage [4]. The absence of remarkable axonal regeneration on electrodiagnostic studies after treatment also supports this hypothesis.
The optimal treatment for primary NL is still under debate. Although various therapeutic approaches, including nerve resection, radiotherapy, and chemotherapy, have been attempted in previous studies, the outcomes have been poor [6,10]. Because of the aggressive nature of the non-germinal center B-cell type DLBCL, we administered R-CHOP with local radiotherapy to our patient. Consequently, satisfactory remission was achieved.
In summary, NL should be considered as a differential diagnosis in patients presenting with paresthesia, pain, and lower limb weakness to avoid misdiagnosis of peripheral neuropathy or lumbar disc pathology. FDG PET/CT is the most sensitive diagnostic tool for disease staging and monitoring treatment response. Electrodiagnostic studies provide additional information regarding the cause of neurological symptoms. If motor weakness persists even after appropriate treatment, rehabilitation should be performed to maintain the muscle bulk, prevent contracture, and aid self-regeneration. Considering its aggressive nature, early diagnosis and appropriate treatment with chemotherapy and radiation therapy should be performed in patients with NL.
Notes
References
1. Gan HK, Azad A, Cher L, Mitchell PL. Neurolymphomatosis: diagnosis, management, and outcomes in patients treated with rituximab. Neuro Oncol. 2010; 12:212–5.

2. Viala K, Béhin A, Maisonobe T, Léger JM, Stojkovic T, Davi F, et al. Neuropathy in lymphoma: a relationship between the pattern of neuropathy, type of lymphoma and prognosis? J Neurol Neurosurg Psychiatry. 2008; 79:778–82.

3. Roncaroli F, Poppi M, Riccioni L, Frank F. Primary non-Hodgkin’s lymphoma of the sciatic nerve followed by localization in the central nervous system: case report and review of the literature. Neurosurgery. 1997; 40:618–22.

4. Jeong J, Kim SW, Sung DH. Neurolymphomatosis: a single-center experience of neuromuscular manifestations, treatments, and outcomes. J Neurol. 2021; 268:851–9.

5. Advani P, Paulus A, Murray P, Jiang L, Goff R, Pooley R, et al. A rare case of primary high-grade large B-cell lymphoma of the sciatic nerve. Clin Lymphoma Myeloma Leuk. 2015; 15:e117–20.

6. Kahraman S, Sabuncuoglu H, Gunhan O, Gurses MA, Sirin S. A rare reason of foot drop caused by primary diffuse large b-cell lymphoma of the sciatic nerve: case report. Acta Neurochir (Wien). 2010; 152:125–8.

7. Priori A, Magno S, Campiglio L, Lovati E, Tagliabue L. Imaging of sciatic lymphoma. Muscle Nerve. 2017; 56:E22–3.

8. Lhermitte J, Trelles J. Neurolymphomatosis péripherique humaine. Presse Med. 1934; 42:289–92.
9. Grisariu S, Avni B, Batchelor TT, van den Bent MJ, Bokstein F, Schiff D, et al. Neurolymphomatosis: an International Primary CNS Lymphoma Collaborative Group report. Blood. 2010; 115:5005–11.

10. Descamps MJ, Barrett L, Groves M, Yung L, Birch R, Murray NM, et al. Primary sciatic nerve lymphoma: a case report and review of the literature. J Neurol Neurosurg Psychiatry. 2006; 77:1087–9.

11. Facchinelli D, Ciliberti E, Stüssi G, Ceriani L, Zucca E. Sciatic pain by neurolymphomatosis as initial presentation of disseminated diffuse large B cell lymphoma involving the testis and the CNS. Hematol Oncol. 2020; 38:197–200.

12. Baehring JM, Damek D, Martin EC, Betensky RA, Hochberg FH. Neurolymphomatosis. Neuro Oncol. 2003; 5:104–15.

13. Quiñones-Hinojosa A, Friedlander RM, Boyer PJ, Batchelor TT, Chiocca EA. Solitary sciatic nerve lymphoma as an initial manifestation of diffuse neurolymphomatosis: case report and review of the literature. J Neurosurg. 2000; 92:165–9.
Fig. 1.
Magnetic resonance images of the lower extremity (A) before and (B) after treatment. (A) An enlarged fusiform mass of approximately 6.3×2.9×2.8 cm (arrows) is seen at the left greater sciatic foramen from the L5 and S1 nerve roots to the sciatic nerve with abnormal progressive gadolinium enhancement and a non-enhancing central portion on axial and coronal T1-weighted images. (B) Markedly decreased size of the enhancing mass (arrow) at the left greater sciatic foramen with mild thickening and residual enhancement of the sciatic nerve after the fourth session of chemotherapy. Left gluteal muscle atrophy (arrowhead) is seen.
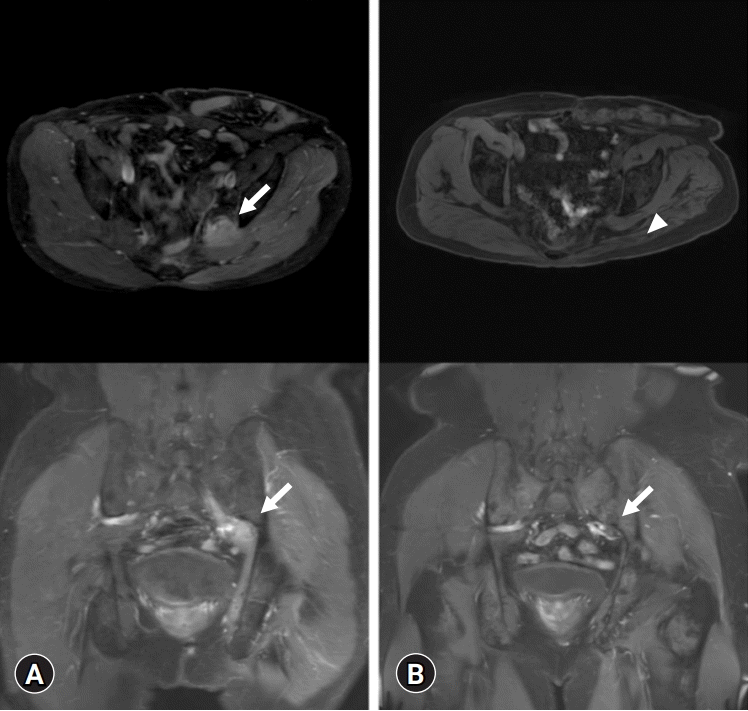
Fig. 2.
Histopathologic findings of the needle biopsied soft tissue adjacent to the left sciatic nerve. (A) Large, irregularly-shaped lymphoid cell proliferation with a diffuse pattern. These atypical lymphoid cells have a large nucleus with clear perinuclear space due to shrunken cytoplasm (hematoxylin and eosin stain, ×400). (B) Immunohistochemical stain shows large lymphoid cells that are positive for CD20 (immunohistochemical stain, x400).
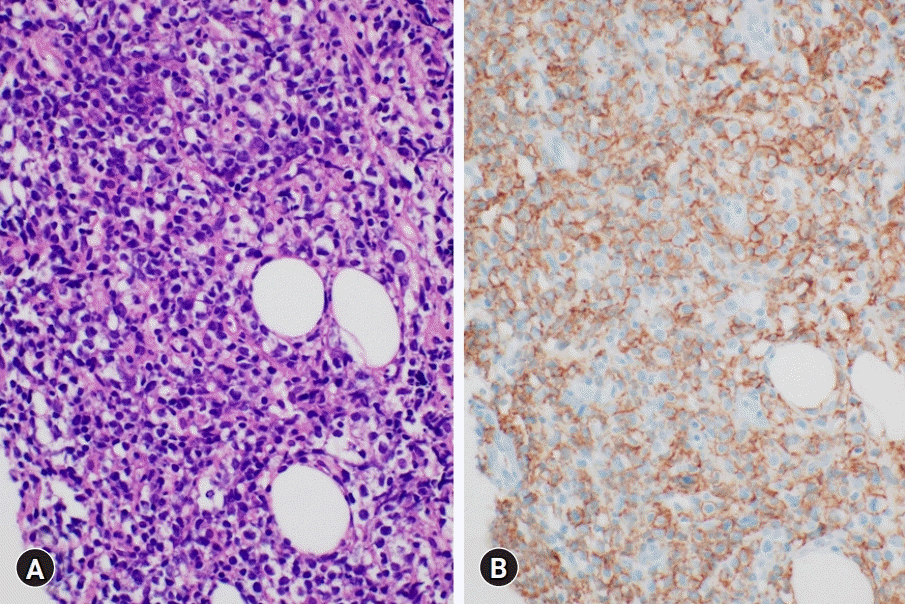
Fig. 3.
Axial and coronal fluorodeoxyglucose positron emission tomography/computed tomography images of the lower extremity (A) before and (B) after treatment. (A) Diffuse increased uptake in the left sciatic nerve and a few hypermetabolic foci (arrows) at the left presacral area and pelvic cavity. (B) Complete metabolic resolution of the left sacral foramen mass and partial metabolic resolution of the hypermetabolic foci at the left presacral area and pelvic cavity after the third session of chemotherapy.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download