This article has been
cited by other articles in ScienceCentral.
Abstract
Immune checkpoint inhibitors (ICIs) have become the main drugs for programmed cell death receptor-1 or ligand-1 expressing non-small cell lung cancer (NSCLC) combined with conventional chemotherapy. ICIs are generally more tolerable than cytotoxic chemotherapies in terms of toxicity, and ICI-related adverse events are mild and manageable. However, these drugs may lead to unexpected severe adverse events such as immune-related hematologic toxicities, which could be life-threatening. Here, a rare case of a pembrolizumab-related adverse event in a patient with NSCLC who showed early-onset hemolytic anemia and recovered by high-dose steroid and a series of plasma exchanges is reported.
Go to :

Keywords: Autoimmune hemolytic anemia, Immune checkpoint inhibitors, Non-small-cell lung carcinoma, Pembrolizumab
Introduction
Efforts to turn on the immune system against cancers have led to the development of immune checkpoint inhibitors (ICIs) targeting programmed cell death receptor-1 or ligand-1 (PD-1/PD-L1) [
1]. Since the first checkpoint molecular inhibitor ipilimumab was approved by the U.S. Food and Drug Administration (FDA), anti-PD-1/PD-L1 therapies are widely used in various cancers to improve survival outcomes, particularly in metastatic non-small cell lung cancer (NSCLC) and melanoma [
2].
Although tumor cells escape immune attack through various complementary mechanisms of immunosuppression, ICIs targeting immunosuppressive molecules, such as cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and PD-1/PD-L1, can reactivate cytotoxic T cells to kill malignant cells [
3]. Meanwhile, ICIs break the balance of the immune system, which may lead to the development of immune-related adverse events (IRAEs) in some patients. Generally, IRAEs can show variable autoimmune manifestations involving the skin, gastrointestinal tract, endocrine system, lung, joints, and many other organs [
4]. Rarely, hematologic manifestations, such as cytopenias, have also been reported in patients with solid tumors and lymphomas during ICI treatment [
5]. Most IRAEs are manageable by suppressing lymphocyte activation with steroids. However, some severe cases require changes in the treatment strategy [
6].
Currently, pembrolizumab has become the first-line standard regimen for metastatic NSCLC with positive PD-L1 expression and negative actionable mutations for targeted therapies [
7]. However, clinicians are faced with unexpected IRAEs and need more experience to overcome IRAEs. This paper reports a rare case of autoimmune hemolytic anemia (AIHA) in a patient with NSCLC receiving a pembrolizumab-containing regimen for metastatic lung cancer.
Go to :

Case
A 70-year-old male newly diagnosed with metastatic NSCLC visited our oncology department in September 2020. The biopsied lung tissue revealed a poorly differentiated adenocarcinoma. Immunohistochemistry analysis showed overexpression of PD-L1 (100% by 22C3 pharmDx assay) and no alterations for targeted therapy including epidermal growth factor receptor, anaplastic lymphoma kinase, or c-ros oncogene 1. The patient’s general condition was good with an Eastern Cooperative Oncology Group performance status of 1, and he had no history of other diseases. He received pembrolizumab plus pemetrexed and cisplatin as first-line therapy for metastatic NSCLC based on international guidelines. His baseline complete blood cell count analysis at the beginning of chemotherapy revealed a white blood cell count of 11,450/μL, hemoglobin (Hb) 13.4 g/dL, and platelet (PLT) count of 218,000/μL.
Two weeks later, he complained of worsening dyspnea and was admitted for evaluation. Results of blood test showed Hb of 5.8 g/dL, total bilirubin of 4.16 mg/dL (direct bilirubin, 0.59 mg/dL and indirect bilirubin, 3.57 mg/dL), depletion of haptoglobin (<10 mg/dL; range, 30–200 mg/dL), and increased serum lactate dehydrogenase (LDH) (478 U/L; range, 140–271 U/L). Over 20% of spherocytes were identified in the peripheral blood smear, and the direct antiglobulin test (DAT) and cold agglutinin test were positive (
Table 1). With an increased reticulocyte count, we considered his severe anemia as AIHA, a rare IRAE of ICIs. The patient was administered prednisolone (1 mg/kg). Despite using high-dose steroids over a week, his LDH continuously increased over 1,000 U/L and PLT count decreased to <100,000/μL without any sign of improvement in hemolytic anemia. Therefore, he was started on prednisolone at 2 mg/kg and underwent plasma exchange five times every other day. Features of hemolytic anemia showed significant improvement after 2 weeks of increased steroid dose and plasma exchange; therefore, the steroid dose was tapered slowly up to 10 mg/day while maintaining the normalized hemoglobin level. The patient then received subsequent conventional chemotherapy with pemetrexed plus cisplatin, and there was no recurrence of hemolysis. The overall treatment and progress are summarized in
Fig. 1.
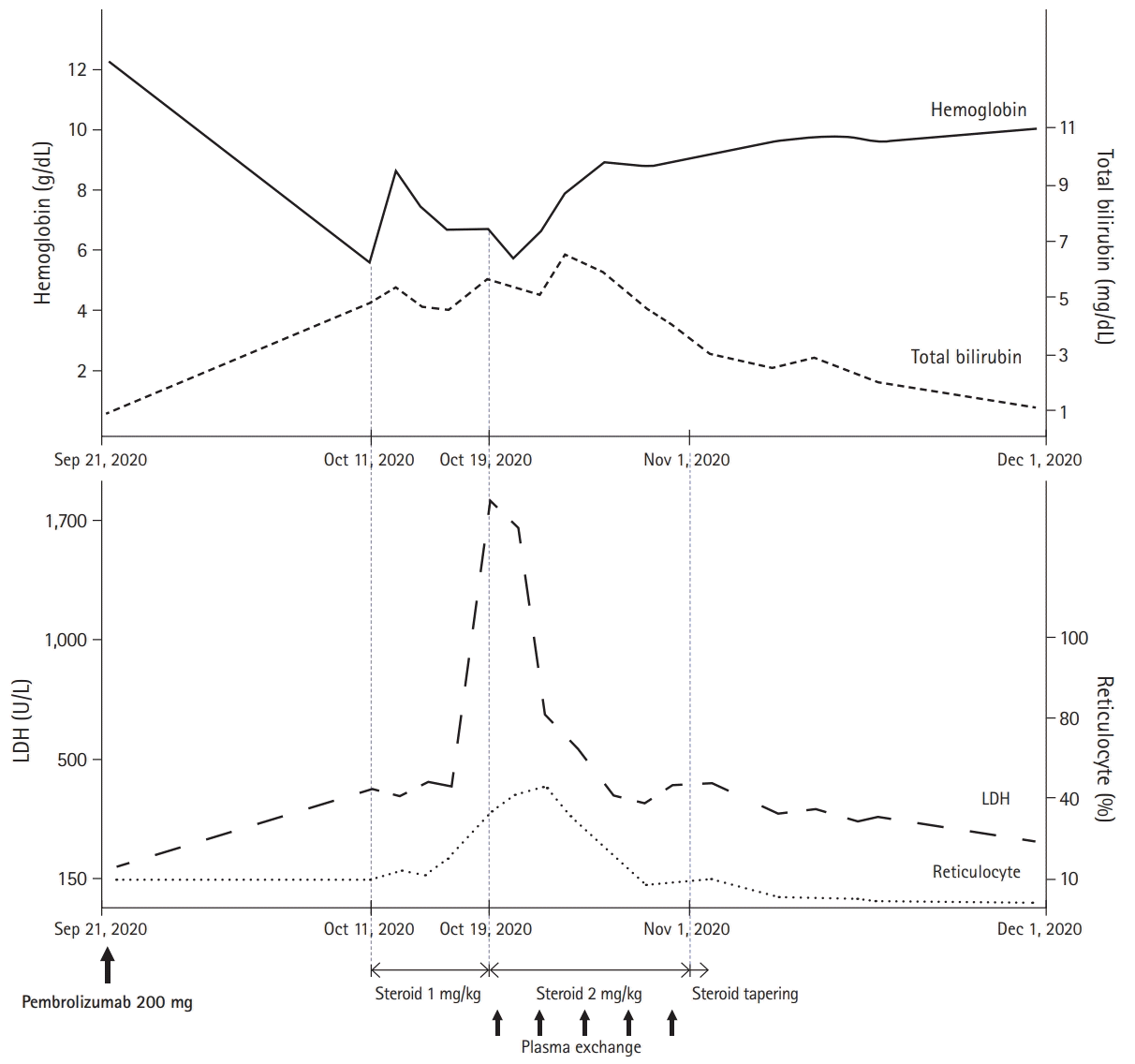 | Fig. 1.Time course of hemoglobin, bilirubin, serum lactate dehydrogenase (LDH), reticulocyte, and treatment. 
|
Table 1.
Results of laboratory investigations corresponding to hemolytic anemia
|
Investigation |
Result (normal range) |
|
Hemoglobin (g/dL) |
5.8 (11–17) |
|
Reticulocytes (×109/L) |
160 (22–139) |
|
Total bilirubin (mg/dL) |
4.16 (0.3–1.2) |
|
Lactate dehydrogenase (U/L) |
478 (140–271) |
|
Haptoglobin (mg/dL) |
<10 (30–200) |
|
Direct antiglobulin test |
Positive |
|
Cold agglutinin |
Positive |

Go to :

Discussion
Previous studies have reported that the frequency of immune-mediated cytopenias is <0.5% in patients treated with ICIs [
8]. According to the details of 68 AIHA cases identified in the FDA database, AIHA was relatively infrequent with pembrolizumab and ipilimumab compared to atezolizumab and nivolumab (
Table 2) [
9]. PD-1 inhibitor-associated AIHA has been reported to usually occur after two to five cycles of treatment compared with 8 to 12 weeks with CTLA-4 inhibitor therapy [
10]. In the described case, the patient showed features of hemolytic anemia with positive DAT and the presence of cold agglutinin after only one cycle of pembrolizumab, including combination chemotherapy. Generally, a positive DAT is an important AIHA feature, and >50% of immune-mediated anemia show positive DAT with immunoglobulin (Ig) G or C3. Cold agglutinin disease (CAD) is another form of AIHA that accounts for 10% to 20% of AIHA. Hemolysis of CAD is primarily extravascular, which is mediated by IgM, cold agglutinin, and complements at low temperatures [
11]. Although pembrolizumab-associated AIHA with cold agglutinin and DAT is rare, a similar case of metastatic lung cancer has been reported [
12].
Table 2.
Proportion of AIHA in the OAEs and characteristics of patients who developed AIHA according to the FDA database
|
Variable |
Ipilimumab |
Nivolumab |
Pembrolizumab |
Atezolizumab |
|
Case of AIHA/OAEa)
|
7/12,631 (0.055) |
43/20,335 (0.211) |
13/8,917 (0.146) |
5/2,021 (0.247) |
|
Age (yr)b)
|
65 (32–68) |
68 (43–85) |
62 (35–82) |
67 (57–69) |
|
Underlying malignancy |
MM: 7 |
LC: 18, MM: 17c), HL: 3c), RCC: 2, Others: 4 |
MM: 7, LC: 5, Others: 1 |
BC: 2, LC: 1, MM: 1, OC: 1 |
|
Other reported hematological adverse event with AIHA |
Agranulocytosis, bicytopenia |
ITP |
ITP, PRCA |
- |

The general recommendations for AIHA management include steroids and consideration of plasma exchange, and the guidelines on the management of ICI-related AIHA are similar. The key mechanism of IRAEs is that ICI therapies disrupt immunological homeostasis and reduce T-cell tolerance [
13]. Therefore, the European Society of Medical Oncology suggests that high-dose corticosteroids and/or other immunosuppressive drugs should be considered in cooperation with a hematologist [
14]. The American Society of Clinical Oncology recommends discontinuation of ICIs and commencement of prednisone (1–2 mg/kg/day) with red blood cell transfusion targeting Hb of 7 to 8 g/dL [
15]. Previous studies have reported that immune-related anemia may be steroid-resistant, and 1 mg/kg of prednisone may be insufficient to resolve hemolysis [
5,
8]. In particular, some cases with nivolumab showed a poor response to steroids, resulting in fatal outcomes. Conversely, some cases with pembrolizumab-related AIHA were relatively not severe and responded to steroids [
9,
16,
17]. In our experience, there was little effect with 1 mg/kg of prednisone, and the features of severe hemolytic anemia began to improve by increasing the prednisone dose to 2 mg/kg/day. Maintaining high-dose steroids over a week with plasma exchange was also important in controlling hemolysis and preventing recurrence.
ICI therapy-associated AIHA is rare, but most cases can be life-threatening. Recently, the number of reported cases has increased with the expansion of ICIs and the number of patients exposed to them. Early recognition is important, and we hope that patients with ICI-related AIHA can receive appropriate management, including high-dose steroid and plasma exchange, through this case report. Further studies with immune cell profiling in patients with hematological IRAEs should be performed to better understand its mechanism and adequate management. In addition, trials including other ICIs with reportedly less hematological IRAEs for patients who experienced immune-related hemolytic anemia should be considered.
Go to :






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download