Abstract
ACKNOWLEDGMENTS
References
Fig. 1.
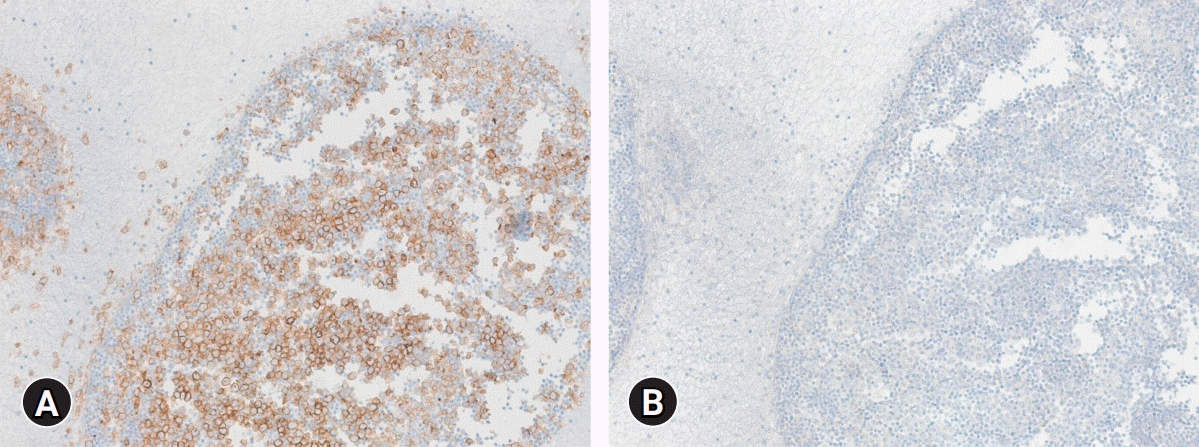
Table 1.
| ALCL deaths from MDRs and literature reported as MDRsa) |
Deaths through 7/6/2019 (n=33) |
||
|---|---|---|---|
| n | %b) | ||
| Age at time of diagnosis (yr) | Median | 52 | |
| Range | 37–83 | ||
| Not specified (no. of reports) | 13 | 39 | |
| Time from the last implant to diagnosis (yr) | Median | 9 | |
| Range | 1–20 | ||
| Not specified (no. of reports) | 23 | 70 | |
| Implant surface | Textured | 15 | 48 |
| Smootha) with history of textured | 1 | 3 | |
| Not specified | 17 | 48 | |
| Implant fill | Silicone | 14 | 42 |
| Saline | 8 | 24 | |
| Not specified | 11 | 33 | |
| Reason for implant | Reconstruction | 5 | 15 |
| Augmentation | 17 | 52 | |
| Not specified | 11 | 33 | |
| Clinical presentation (breast)c) | Seroma | 6 | 18 |
| Breast swelling/pain | 3 | 9 | |
| Capsular contracture | 1 | 3 | |
| Peri-implant mass/lump | 13 | 39 | |
| Others | 7 | 21 | |
| Not specified | 7 | 21 | |
| Anaplastic lymphoma kinase | Positive | 0 | 0 |
| Negative | 12 | 36 | |
| Not specified | 21 | 64 | |
| CD30 statusd) | Positive | 12 | 36 |
| Negative | 0 | 0 | |
| Not specified | 21 | 64 | |
| Implant manufacturer | Allergan | 12 | 36 |
| Mentor | 1 | 3 | |
| Unknown | 20 | 61 | |
| Reporter country: US or OUSe) | US | 12 | 36 |
| OUS | 21 | 64 | |
| Not specified | 0 | 0 | |
US, the United States; MDR, medical device report; ALCL, associated anaplastic large-cell lymphoma; OUS, outside the US.
b) Percentage in terms of the total 33 deaths. There are no reports of deaths associated with tissue expanders.
c) MDRs sometimes list more than one clinical presentation, e.g., seroma and peri-implant mass/lump, in which two presentations were counted.
d) CD30 is a cell membrane protein associated with diagnosis of classic Hodgkin’s lymphoma and breast implant-ALCL.
e) US/OUS is counted as the country reported in the narrative or the recorded reporter’s country in the MedWatch form.
Adapted from the materials of U.S. Food and Drug Administration [6].
Table 2.
| Unique ALCL casesa) |
Cases through 9/30/2018 (n=457) |
Cases through 7/6/2019 (n=573) |
|||
|---|---|---|---|---|---|
| n | %b) | n | %c) | ||
| Age at time of diagnosis (yr) | Median | 53 | - | 53 | - |
| Range | 27–90 | - | 27–90 | - | |
| Not specified (no. of reports) | 111 | 24 | 161 | 28 | |
| Time from the last implant to diagnosis (yr) | Median | 9 | - | 8 | - |
| Range | 0–34 | - | 0–34 | - | |
| Not specified (no. of reports) | 110 | 24 | 169 | 29 | |
| Implant surface | Textured | 310 | 68 | 385 | 67 |
| Smooth | 24 | 5 | 26d) | 5 | |
| Not specified | 123 | 27 | 162 | 28 | |
| Implant fill | Silicone | 274 | 60 | 343 | 60 |
| Saline | 183 | 40 | 197 | 34 | |
| Not specified | 0 | 0 | 33 | 6 | |
| Reason for implant | Reconstruction | 108 | 24 | 115 | 20 |
| Augmentation | 104 | 23 | 111 | 19 | |
| Not specified | 245 | 54 | 347 | 61 | |
| Clinical presentation (breast)e) | Seroma | 266 | 58 | 302 | 53 |
| Breast swelling/pain | 135 | 30 | 150 | 26 | |
| Capsular contracture | 69 | 15 | 73 | 13 | |
| Peri-implant mass/lump | 82 | 18 | 94 | 16 | |
| Others | 43 | 9 | 56 | 10 | |
| Not specified | 105 | 23 | 147 | 26 | |
| Anaplastic lymphoma kinase | Positive | 0 | 0 | 0 | 0 |
| Negative | 229 | 50 | 255 | 45 | |
| Not specified | 228 | 50 | 318 | 55 | |
| CD30 statusf) | Positive | 215 | 47 | 246 | 43 |
| Negative | 0 | 0 | 0 | 0 | |
| Not specified | 242 | 53 | 327 | 57 | |
| Implant manufacturer | Allergan includes McGhan, Inamed | 386 | 84 | 481 | 84 |
| Mentor | 36 | 8 | 38 | 7 | |
| Sientra | 2 | 0.4 | 6 | 1 | |
| Other manufacturerg) | 5 | 1 | 6 | 1 | |
| Unknown manufacturer | 28 | 6 | 42 | 7 | |
| Reporter country: US or OUSh) | US | 276 | 48 | 320 | 56 |
| OUS | 181 | 32 | 253 | 44 | |
| Not specified | 0 | 0 | 0 | 0 | |
US, the United States; MDR, medical device report; ALCL, associated anaplastic large-cell lymphoma; OUS, outside the US.
d) In the 26 cases of smooth implants, 12 have unknown prior history of implants, seven have a history of textured implants, and seven have a history of prior implants with an unknown texture. There are no reports of cases associated with tissue expanders.
e) MDRs sometimes list more than one clinical presentation, e.g., seroma and peri-implant mass/lump, in which two presentations were counted.
f) CD30 is a cell membrane protein associated with diagnosis of classic Hodgkin’s lymphoma and BIA-ALCL.
g) Other manufacturers include: Bristol Myers Squib, Nagor, Polytech Silimed, Silimed, and Sientra/Silimed.
h) US/OUS is counted as the recorded reporter’s country in the MedWatch form, or if the event was noted to be from a foreign source in box G3 of the MedWatch form. Please note that the reporter country may not reflect the country where the event occurred or the country where the device is marketed.
Adapted from the materials of U.S. Food and Drug Administration [6].




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download