Abstract
Notes
Ethical statements
This report was approved by the Institutional Review Board (IRB) of the Yeungnam University Hospital (IRB No: YUMC 2020-07-038). Written informed consent was obtained from the patient for the publication of this case report and the accompanying images.
Author contributions
Conceptualization: all authors; Data curation: SML, YTL, KMJ, MJG, JHL; Formal analysis and Supervision: JML; Funding acquisition and Validation: JML; Methodology: SML, YTL, KMJ, MJG, JHL; Investigation: SML; Resources: YTL, KMJ, MJG, JHL; Writing-original draft: all authors; Writing-review & editing: all authors.
References
Fig. 1.
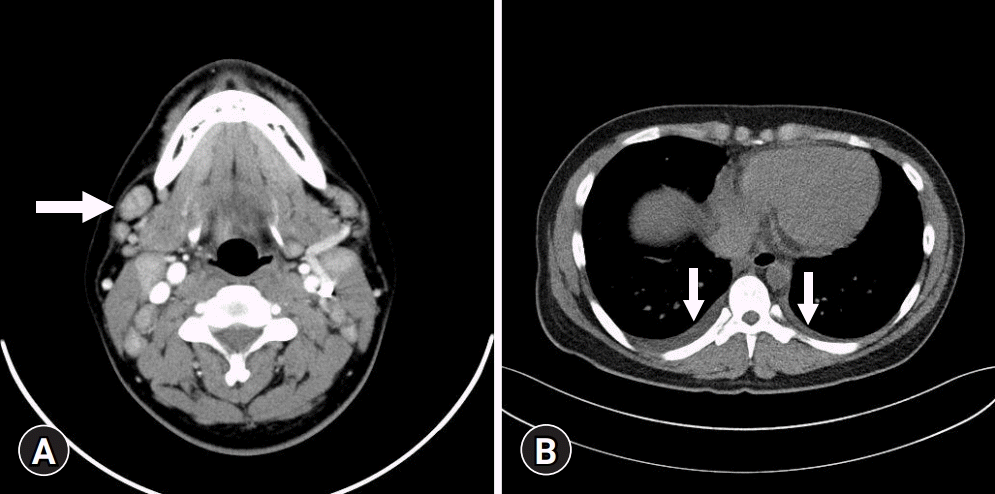
Fig. 2.
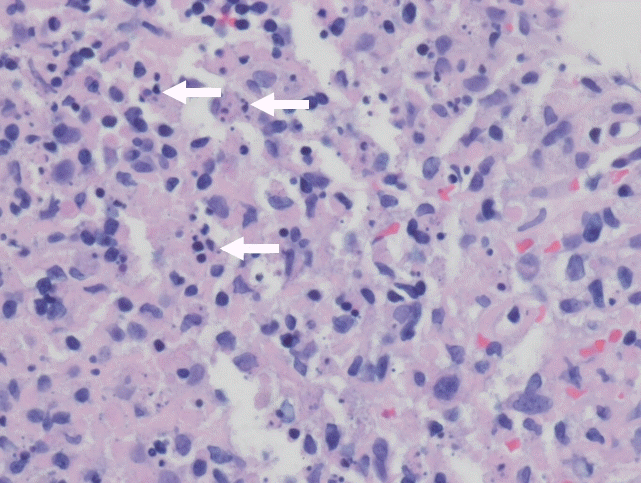
Fig. 3.
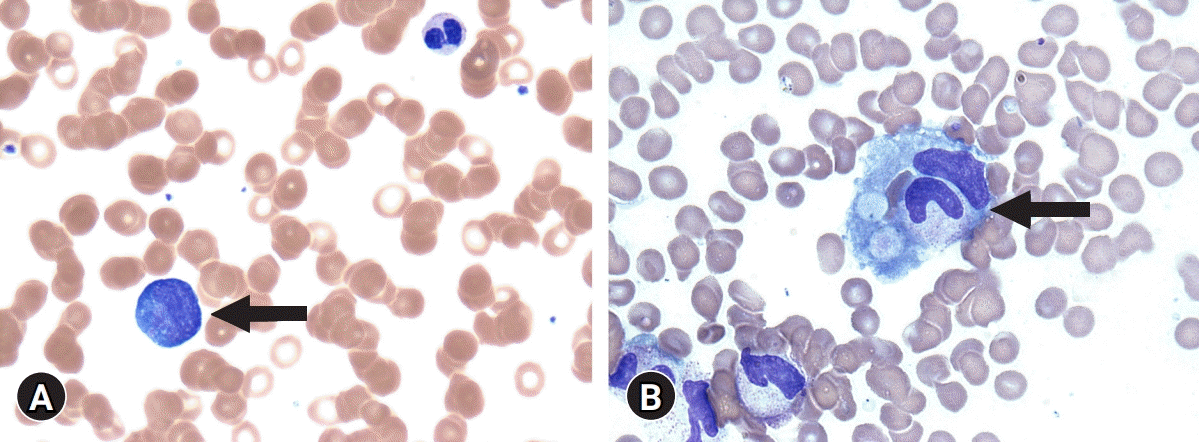
Table 1.
| Year | Study | No. of patients | Age (yr) | Sex | Country | Race | Symptom | Treatment | Outcome | Remark |
|---|---|---|---|---|---|---|---|---|---|---|
| 2000 | Chen et al. [5] | 2 | 14 | M | Taiwan | Asian | Fever, fatigue, cervical lymph node swelling | IVIG | CR | NA |
| 10 | F | Taiwan | Asian | Fever, cervical lymph node swelling | Steroid, IVIG | CR | NA | |||
| 2000 | Kelly et al. [6] | 1 | 17 | F | United Kingdom | Asian | Fever, cervical lymphadenopathy | IVIG | CR | NA |
| 2003 | Kim et al. [7] | 1 | 13 | F | Korea | Asian | Fever, seizure, cervical lymph node swelling | Steroid, IVIG | CR | NA |
| 2007 | Lin et al. [8] | 1 | 13 | M | Japan | Asian | Fever, axillary and inguinal lymphadenopathy | Steroid | CR | NA |
| 2007 | Khan et al. [9] | 1 | 40 | M | Qatar | Asian | Fever, cervical lymphadenopathy | NSAIDs | CR | NA |
| 2008 | Lim et al. [10] | 5 | 12 | M | Korea | Asian | Fever, cervical lymphadenopathy | Steroid | CR | Recurred after 2 yr |
| 14 | M | Korea | Asian | Fever, multiple lymphadenopathy | Steroid, IVIG, ACV, VP16 | CR | EBV, recurred after 7 yr | |||
| 5 | F | Korea | Asian | Fever, multiple lymphadenopathy | Steroid, VP16 | NR, died | NA | |||
| 14 | F | Korea | Asian | Fever, cervical lymphadenopathy | Steroid, IVIG, VP16, CyA | CR | NA | |||
| 8 | M | Korea | Asian | Fever, multiple lymphadenopathy | Steroid | CR | EBV | |||
| 2008 | Kampitak [11] | 1 | 50 | M | Thailand | Asian | Fever, fatigue, myalgia, multiple lymphadenopathy, seizure | Steroid, immunosuppressive therapy | NR, died | SLE |
| 2009 | Byoun et al. [12] | 1 | 21 | F | Korea | Asian | Fever, cervical lymphadenopathy | Steroid, ACV | CR | EBV |
| 2010 | Lee et al. [13] | 1 | 16 | M | Taiwan | Asian | Fever, cervical and axillary lymphadenopathy | Antibiotics | CR | EBV |
| 2011 | Kim et al. [14] | 1 | 18 | M | Korea | Asian | Fever, cervical lymphadenopathy | Steroid, IVIG, VP16, CyA | CR | NA |
| 2011 | Kim et al. [15] | 1 | 0.75 | M | Korea | Asian | Fever, cervical lymphadenopathy | Antibiotics | CR | NA |
| 2013 | Koga et al. [16] | 1 | 21 | M | Japan | Asian | Fever, cervical lymphadenopathy, erythema | Steroid | CR | Sweet’s disease |
| 2016 | Sykes et al. [17] | 1 | 16 | F | United States | NA | Fever, joint pain, fatigue | Antibiotics | CR | NA |
| 2016 | Nishiwaki et al. [18] | 1 | 30 | M | Japan | Asian | Fever, sore throat | Steroid | CR | NA |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download