Abstract
Papillary fibroelastomas are the second most common primary cardiac tumor in adults. Over 80% of fibroelastomas occur on the cardiac valves, usually on the left side of the heart, while the remaining lesions are typically scattered throughout the atria and ventricles. Although the optimal timing for surgery is controversial and depends on tumor size and location, prompt surgical resection is warranted in patients at high risk of embolism. A tumor on the cardiac valve can be removed using the slicing excision technique without leaflet injury. Here we present two cases of papillary fibroelastomas occurring on the ventricular surface of the aortic valve and in the right ventricle.
Cardiac papillary fibroelastoma (PFE), the second most common primary cardiac tumor in adults, has an incidence of 0.02% in every one million autopsies [1]. Its appearance is often likened to sea anemones with frond-like arms emanating from a stalked central core.
In two large studies of PFE patients a mean age of 60 years, the incidence was higher in men (55%); at the time of discovery, the tumors were 2 to 70 mm and the average size was 9 mm [1,2]. Cardiac PFEs usually occur in the heart valve; more than 80% of cases were noted on the left-sided heart valves (aortic, 36%; mitral, 29%; tricuspid, 11%; and pulmonary, 7%), whereas the other lesions were usually scattered throughout the atria and ventricles. Multiple tumors were present in 9% of patients [1,2]. Of PFE cases, 30% were detected accidentally in asymptomatic patients, whereas the other patients presented with stroke, myocardial infarction, and systemic or pulmonary embolic events associated with tumor embolism [1,2]. This case report discusses the ability of early intervention in unusually located PFEs to prevent the occurrence of an embolic event and analyzes whether valve-sparing surgery can be performed safely in cases of valvular involvement in tumors without a risk of recurrence after total excision. Here we present two cases of PFE located at unusual sites.
This study was approved by the Institutional Review Board (IRB) of Sanggye Paik Hospital (IRB No: SGPAIK 2019-12-008). Written informed consents were obtained from the patients for publication of this case report and accompanying images.
A 61-year-old woman visited the outpatient clinic for the treatment of a cardiac mass discovered during the workup performed prior to knee arthroplasty surgery. Transthoracic echocardiography showed a 0.8×0.9-cm mobile mass on the ventricular surface of the noncoronary cusp of the aortic valve without valvular dysfunction (Fig. 1A, 1B). Computed tomography also revealed a mass-like lesion between the left ventricular outflow tract and the aortic valve (Fig. 1C). Based on these reports, a PFE was suspected. Surgery was planned immediately because of the hypermobility of the mass at the aortic valve that could potentially cause a fatal cerebral infarction.
The aortic valve was morphologically normal in appearance (Fig. 2A, 2B), and the tumor was removed using the slicing excision technique. After tumor removal, the aortic valve showed normal coaptation without leaflet injury, while postoperative transthoracic echocardiography showed normal function of the aortic valve. The pathologic examination showed findings typical of PFE (Fig. 3). After being discharged from the hospital in a stable condition, the patient was followed up at the outpatient clinic for 1 year without tumor recurrence.
A 65-year-old man was referred for aortic stenosis with a mass in the right ventricle discovered during the preoperative workup for a right humeral fracture. Transthoracic echocardiography showed severe aortic stenosis with a bicuspid aortic valve and a 1.5-cm hypermobile mass attached to the apex of the right ventricle (Fig. 1D, 1E). Computed tomography showed an aneurysmal change of the ascending aorta (4.7 cm) and a mass lesion in the right ventricle (Fig. 1F).
The tumor was removed concomitantly with replacement of the aortic valve and ascending aorta. The tumor was attached to the myocardial wall at the apex of the right ventricle (Fig. 2C). Immersion of the tumor in water after resection revealed the typical sea anemone appearance (Fig. 2D). After being discharged without complications, the patient was followed up at the outpatient clinic for 18 months without tumor recurrence.
Although PFE is the second most common cardiac tumor, its incidence is very low. It is difficult to diagnose before embolic events occur. This study presented two PFE cases that were referred to the medical facility after masses were discovered during preoperative workups for noncardiac surgeries.
In these cases, the tumors were located at unusual sites. In case 1, the tumor was attached to the aortic valve, and the mobile mass had grown to 9 mm on the ventricular surface of the leaflet, where it was subjected to high blood pressure and fast blood flow. The tumor in case 2 also occurred at an unusual site, attaching to the endocardium of the right ventricular apex. Although both masses were prone to detachment due to the blood flow, they were discovered before any embolic events occurred.
Echocardiography is a presumptive method for the diagnosis of PFE. However, in both cases, computed tomography showed a clear mass lesion in the heart; these findings might be helpful for screening patients with or without embolic events, and the early detection of PFE is possible through careful consideration of the computed tomography findings.
Decisions regarding the surgical excision of PFE depend on its size, location, and mobility as well as the potential risk of embolism. The excision of an isolated right-sided PFE is indicated only in cases of large mobile tumors that can cause pulmonary embolism. When the tumor is located at the left side of the heart, careful observation is needed if there are no symptoms, the size is less than 1 cm, and the tumor is nonmobile; however, early surgery is required in the case of an embolic event and if the mobile tumor is larger than 1 cm [1,2].
However, some surgeons consider early surgery mandatory because of the potential risk of embolic complications, and valve-sparing surgery can be performed safely in cases of valvular involvement in tumors without a risk of recurrence after total excision [3,4]. According to the results of a comparative study including PFE patients split into a surgically treated group and a group that was kept only under observation with aspirin, the incidence of cerebral vascular accident and mortality rates were higher in the latter [5].
In case 1, the stalk on the ventricular surface of the aortic valve was not firmly attached to the normal tissue and could be easily excised using the slicing technique. After tumor excision, there was no significant damage to the remnant leaflet tissue; thus, no valve repair was required. Additional valve repair is required in the case of tissue defects after wide excision, and valve replacement is inevitable when the leaflet is completely excised.
Even when a tumor is benign, if it has a high possibility of recurrence, extensive resection of the area around the stalk is required. However, in the case of PFE, recurrence is unknown. Hence, after excision, the surgeon can distinguish it from other tumors by immersing it in water and observing the typical sea anemone appearance before submitting it for pathological examination. Therefore, aortic valve-sparing procedures can be the first option for treating tumors on the aortic valve.
In these two cases, both tumors were mobile with small stalks and at high risk of detachment due to the blood flow, especially the tumor in case 1. Despite its small size, the early surgical excision of the mobile tumor located in the left side of the heart can prevent the fatal sequelae of cerebral embolism. Tumors larger than 1 cm located in the right side of the heart should also be considered for excision to prevent pulmonary embolism.
PFE is not generally known to recur after excision; however, one case of recurrence was reported recently, so surgeons should be careful about this possibility in the future [6]. However, PFE recurrence is extremely rare compared to other primary cardiac tumors.
In conclusion, PFE is a rare tumor characterized by a small stalk that can be easily detached through blood flow and cause fatal embolism. Its early diagnosis is possible based on a visible shadow on computed tomography followed by echocardiography. Depending on tumor size and location, fatal embolism can be prevented by appropriate early surgical treatment.
References
1. Gowda RM, Khan IA, Nair CK, Mehta NJ, Vasavada BC, Sacchi TJ. Cardiac papillary fibroelastoma: a comprehensive analysis of 725 cases. Am Heart J. 2003; 146:404–10.

2. Sun JP, Asher CR, Yang XS, Cheng GG, Scalia GM, Massed AG, et al. Clinical and echocardiographic characteristics of papillary fibroelastomas: a retrospective and prospective study in 162 patients. Circulation. 2001; 103:2687–93.

3. Grinda JM, Couetil JP, Chauvaud S, D'Attellis N, Berrebi A, Fabiani JN, et al. Cardiac valve papillary fibroelastoma: surgical excision for revealed or potential embolization. J Thorac Cardiovasc Surg. 1999; 117:106–10.

4. Ngaage DL, Mullany CJ, Daly RC, Dearani JA, Edwards WD, Tazelaar HD, et al. Surgical treatment of cardiac papillary fibroelastoma: a single center experience with eighty-eight patients. Ann Thorac Surg. 2005; 80:1712–8.

Fig. 1.
Radiological findings of case 1 (A–C) and case 2 (D–F). (A, B) Preoperative echocardiograhy shows a mobile mass (arrow) on the ventricular surface of the noncoronary cusp of the aortic valve. (C) Computed tomography image shows a mass lesion (arrow) on the aortic valve. (D, E) Preoperative echocardiography shows a round mass (arrow) in the right ventricle. (F) Computed tomography shows a mass lesion in the right ventricle (arrow).
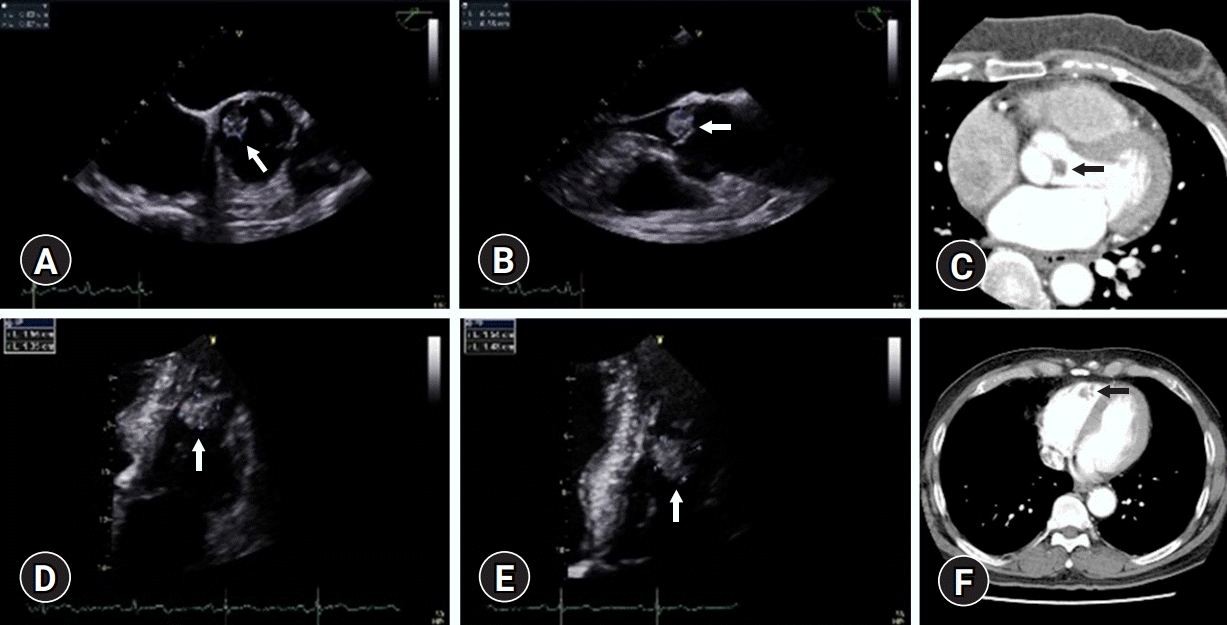
Fig. 2.
Operative and gross findings of case 1 (A, B) and case 2 (C–E). (A) The mass is morphologically round and attached to the ventricular surface of the noncoronary cusp of the aortic valve (arrow). (B) A pinkish round mass measures 0.9 cm in the largest dimension. One tip shows a piece of whitish fibrous tissue that might be attached to the aortic valve. (C) The mass is morphologically round and attached to the right ventricular wall (arrow). (D) Immersion in water after resection reveals a typical sea anemone appearance. (E) A whitish papillary mass measures 1.5 cm in the largest dimension. (E) One tip shows a piece of brownish muscular tissue that might be attached to the myocardial wall.
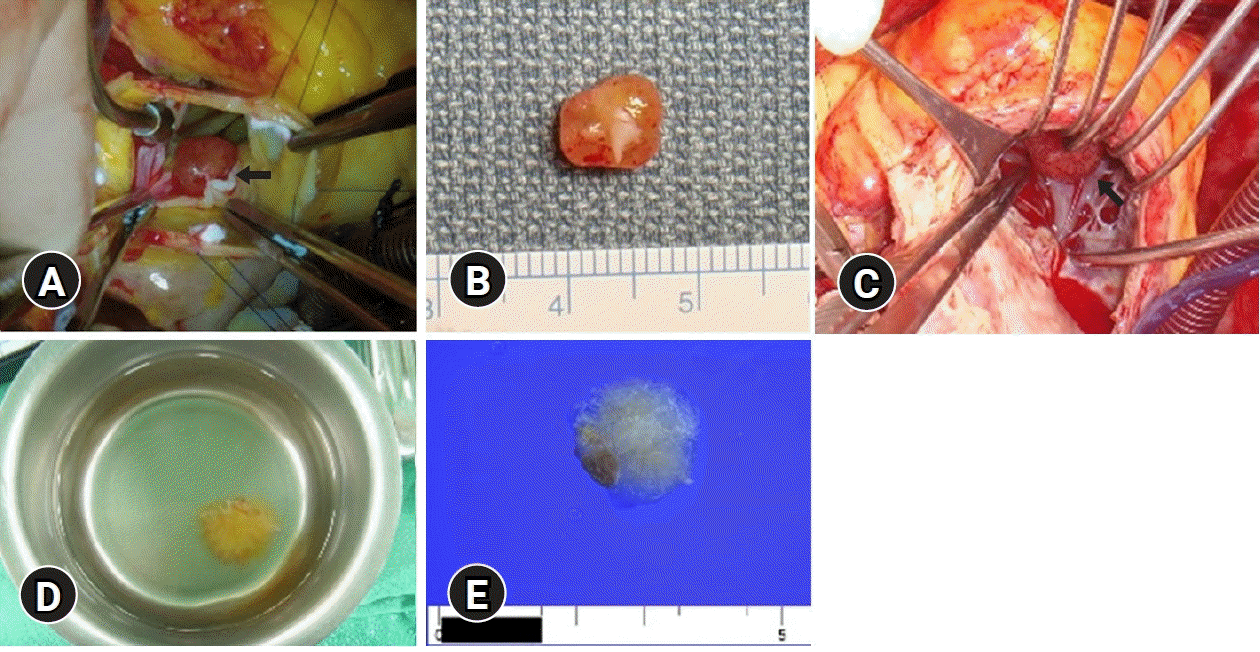
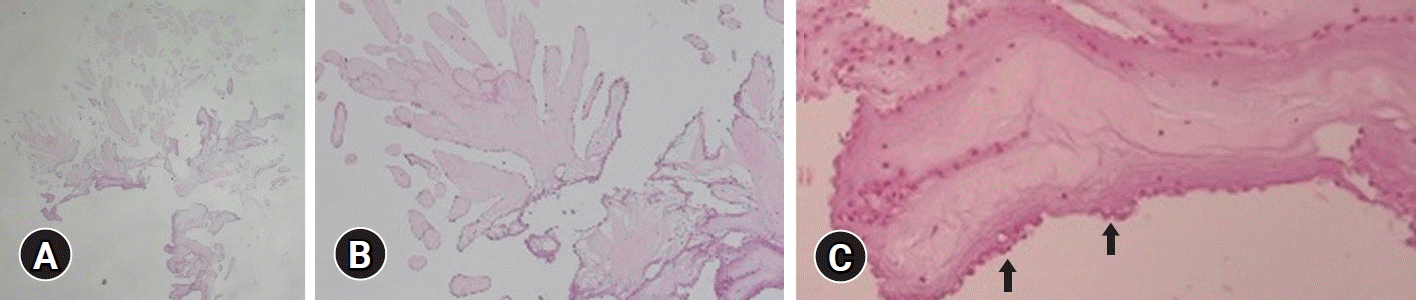
Fig. 3.
Histopathological findings. (A) A papillary mass at low power shows numerous arborizing fronds from a common central stalk (hematoxylin and eosin [H&E] stain, ×10). (B) The papillae consist of avascular fronds with hyalinized stroma and partly denuded lining cells (H&E stain, ×40). (C) The fronds are coated by a single endothelial layer (arrows) (H&E stain, ×100).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download