Materials and methods
This study was approved by the Institutional Review Board (IRB) of Yeungnam University Hospital (IRB No: 2016-06-022). Informed consent was obtained from all individual participants included in the study.
1. Study population
We used data from a previous study [
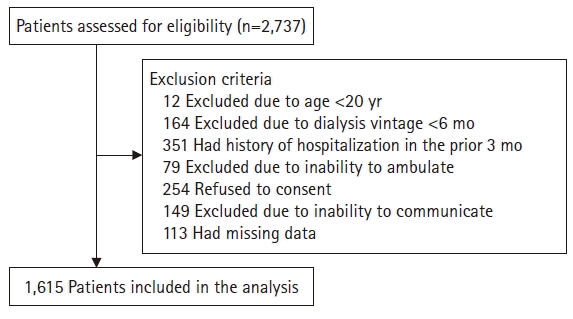
5]. Briefly, a total of 2,737 study participants underwent maintenance dialysis and were enrolled from 27 dialysis centers between July and December 2012. Patients were excluded for the following reasons: age <20 years (n=12), dialysis vintage <6 months (n=164), a history of hospitalization in the prior 3 months for any other than an HD vascular access (n=351), inability to walk with or without an assistive device (n=79), inability to communicate with the interviewer (n=149), refusal to provide informed consent (n=254), or insufficient data (n=113). Finally, a total of 1,615 patients were recruited into our study (
Fig. 1; 1,249 and 366 patients underwent HD and PD, respectively). The participants were recruited between July and December 2012; the follow-up ended in March 2014. During the follow-up period, transfer to other hospitals, change of dialysis modality, kidney transplantation, or follow-up loss was considered as censored data.
2. Study variables
In our study, all variables except patient survival and hospitalization rates were defined using findings at the enrollment. Our study examined age, sex, body mass index (BMI, kg/m2), DM, coronary artery disease (CAD), cerebrovascular disease (CVD), dialysis vintage, and education level, as well as hemoglobin (mg/dL), serum albumin (g/dL), blood urea nitrogen (BUN, mg/dL), creatinine (mg/dL), calcium (mg/dL), phosphorus (mg/dL), total cholesterol (mg/dL), high-sensitivity C-reactive protein (hs-CRP, mg/dL), and intact-parathyroid hormone (i-PTH, pg/mL) levels. DM was defined as a self-reported history and medical record of its diagnosis, or a fasting glucose level of ≥126 mg/dL. CAD was defined as a self-reported history and medical record of angina, myocardial infarction, or congestive heart failure. CVD was defined as a self-reported history and medical record of stroke.
3. Assessment of disability and clinical outcomes
We evaluated disability by using four questions on activities of daily living (ADLs) concerning whether help was needed for feeding, dressing/undressing, getting in/out of bed, or taking a bath/shower [
6]. Each question required one of three responses: no help, some help, or full help. We divided the patients into three groups: Non-D, (non-disabled, none of the four ADL domains required help), Mild-D (mildly disabled, one ADL domain required some or full help), or MS-D (moderate to severely disabled, two or more ADL domains required some or full help).
All data on mortality and hospitalization rates were retrieved from medical records up to March 2014 (mean follow-up duration, 484±113 days). If a patient with HD was admitted for a vascular access-related problem, the hospitalization was not considered as a significant one. We evaluated exhaustion using the vitality (VT) component in HRQoL scales, which consist of four questions: “Did you feel full of pep?”, “Did you have a lot of energy?”, “Did you feel worn out?”, and “Did you feel tired?” Exhaustion was defined as mean vital score <5 on four questions [
5]. We defined a fall as an event that resulted in a participant coming to rest unintentionally on the ground or a lower level with or without losing consciousness during the last 1 year. Serious falls were defined as head injury requiring hospitalization, joint dislocations, severe sprains, or a laceration requiring sutures [
7]. We identified numbers of participants with fall history and total numbers of falls and/or numbers of serious falls.
Exercise was classified using the WHO definition [
8]. Physical activity was defined as the presence of regular exercise during leisure time for the past 3 months. Exercise was defined as engaging in moderate activity for >30 minutes/day for 5 days a week or at a high intensity for >20 minutes/day for 3 days a week. Frailty was defined using modified criteria from the study by Johansen et al. [
9]. Johansen’s criteria include four components (slowness/weakness, 2 points for physical functioning scale <75; poor endurance/exhaustion, 1 point for VT scale <55; physical inactivity, 1 point for physically active <1 day/week during their leisure time for the past 3 months; and unintentional weight loss, 1 point for unintentional body weight loss >4.5 kg or 5% of baseline value over the past year). A summed score ≥3 is defined as frailty.
4. Health-related quality of life assessment
The Kidney Disease Quality of Life-Short Form (KDQOL-SF) Korean version 1.3 was used to evaluate HRQoL [
10]. This questionnaire includes the 36-Item Short Form Health Survey (SF-36)with eight domains (physical functioning, role limitations due to physical health problems, body pain, general health, VT, social functioning, role limitations due to emotional problems, and mental health) and an overall health rating. It also includes a kidney disease-specific scale with 11 items (symptom/problems [SP], effects of kidney disease [EKD], burden of kidney disease [BKD], work status [WS], cognitive function [CF], quality of social interaction [QSI], sexual function [SeF], sleep, social support [SS], patient satisfaction [PS], and dialysis staff encouragement [DSE]). Each domain was scored from 0 to 100. The physical component scale (PCS) and mental component scale (MCS) scores were also calculated from the SF-36 scale [
11].
5. Statistical analyses
IBM SPSS Statistics version 23 (IBM Corp., Armonk, NY, USA) was used for analyses. Our data were expressed as the mean±standard deviation (SD) or standard error (SE) for continuous data and as numbers (percentage) for categorical data. Pearson chi-square test was used to analyze categorical data. One-way analysis of variance, followed by a post-hoc Tukey comparison, was used to analyze continuous data. The Kaplan-Meier log-rank test and Cox regression analysis were used to compare survival estimates. Multivariate methodology was used for the analysis of covariance or Cox regression analysis. The covariates included age, sex, BMI, DM, CAD, CVD, dialysis vintage, dialysis modality, education level, and levels of hemoglobin, serum albumin, BUN, serum creatinine, calcium, phosphorus, i-PTH, total cholesterol, and hs-CRP. Logistic regression analysis was used to estimate the odds ratios (ORs) and 95% confidence intervals (CIs), which were then used to determine the correlation between falls and the groups. The level of statistical significance was set at p<0.05.
Results
1. Clinical characteristics
The numbers of participants in the Non-D, Mild-D, and MS-D groups were 1,312, 163, and 140, respectively (
Table 1), and the prevalence of disability was 18.8% in our cohort. Patients in the MS-D group were the oldest among those in the three groups. The proportion of those with comorbidities (DM, CAD, and CVD) increased as the grade of disability increased. The Non-D group had the highest proportion of those on HD. Serum albumin, creatinine, and phosphorus levels in the MS-D group were lower than those in the Non-D group. The mean follow-up duration was 484±113 days. The trend showed lesser follow-up duration in the Mild-D group than in the other groups (
p=0.046 for trends;
p=0.037 for Non-D vs. Mild-D;
p=0.998 for Non-D vs. MS-D;
p=0.161 for Mild-D vs. MS-D).
2. Evaluation of falls, frailty, and exhaustion according to disability
The numbers of participants who had a fall during the past 1 year were 199 (15.2%), 42 (25.8%), and 44 (31.4%) in the Non-D, Mild-D, and MS-D groups, respectively (p<0.001). In addition, the numbers of participants who had a serious fall during the past 1 year were 35 (2.7%), 5 (3.1%), and 6 (4.3%) in the Non-D, Mild-D, and MS-D groups, respectively (p<0.001). The total numbers of falls during the past 1 year were 0.30±1.51, 0.42±0.84, and 0.57±1.01 in the Non-D, Mild-D, and MS-D groups, respectively (p=0.075).
Univariate logistic regression analysis showed that the ORs of falls in the Mild-D and MS-D groups were 1.94 (95% CI, 1.33–2.85; p=0.001) and 2.62 (95% CI, 2.62–3.85; p<0.001), respectively, relative to the Non-D group. The OR of falls in the MS-D group was 1.35 (95% CI, 0.82–2.22; p=0.238), relative to the Mild-D group. Multivariate logistic regression analysis showed that the ORs of falls in the Mild-D and MS-D groups were 1.62 (95% CI, 0.97–2.70; p=0.066) and 2.03 (95% CI, 1.18–3.49; p=0.011), respectively, relative to the Non-D group. The OR of falls in the MS-D group was 1.38 (95% CI, 0.68–2.81; p=0.369), relative to the Mild-D group.
The numbers of participants with frailty in the Non-D, Mild-D, and MS-D groups were 381 (29.0%), 84 (51.5%), and 93 (66.4%), respectively (p<0.001). Additionally, the numbers of participants with exhaustion in the Non-D, Mild-D, and MS-D groups were 844 (64.3%), 112 (68.7%), and 114 (81.4%), respectively (p<0.001). The numbers of participants who performed exercise in the Non-D, Mild-D, and MS-D groups were 313 (23.9%), 30 (18.4%), and 20 (14.3%), respectively (p=0.023).
3. Evaluation of health-related quality of life scales, hospitalization, and survival according to disability
Table 2 shows differences in the HRQoL scales according to disability. On both univariate and multivariate analyses, the PCS and MCS scores decreased as the grade of disability increased. Among kidney disease-specific scales, SP, EKD, BKD, WS, CF, QSI, sleep, and PS were associated with the grade of disability on both univariate and multivariate analyses.
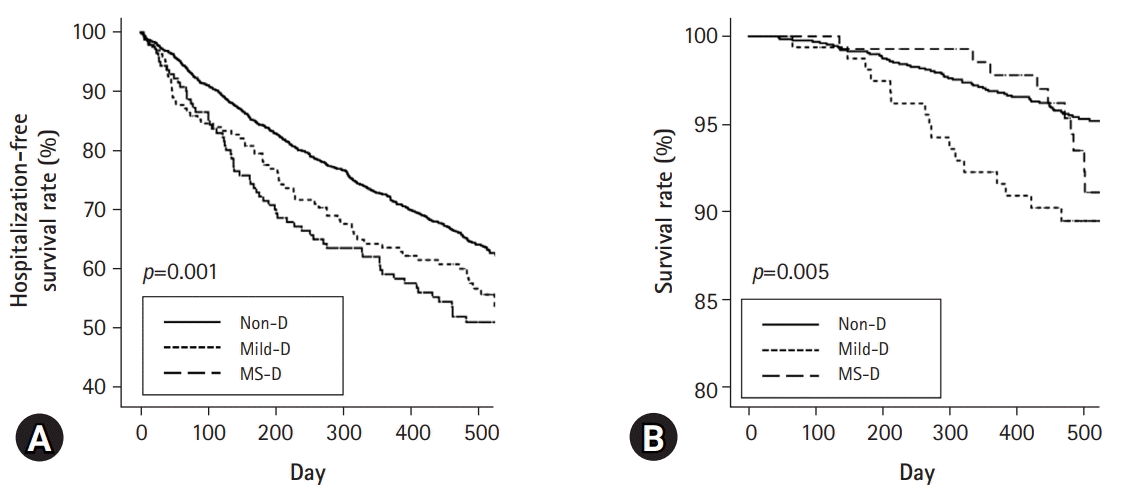
Kaplan-Meier analysis showed that those in the Mild-D and MS-D groups had poor first-hospitalization-free survival compared with those in the Non-D group (
Fig. 2A). The Non-D group had better patient survival compared with the Mild-D group, but there were no significant differences in survival between the Non-D and MS-D groups or the Mild-D and MS-D groups (
Fig. 2B). The total numbers of deaths during follow-up in the Non-D, Mild-D, and MS-D groups were 59 (4.5%), 16 (9.8%), and 11 (7.9%), respectively (
p=0.007), which was lower in the Non-D group than in the other groups. The most common cause of deaths in the Non-D and Mild-D groups was CAD (15 [25.4%] in Non-D group and 4 [25.0%] in Mild-D group), which that in the MS-D group was infection (5 [45.5%]). Multivariate Cox regression analysis showed that the Mild-D group had a higher hazard ratio relative to the Non-D group (
Table 3). Although statistical significance was not observed, the MS-D group also had a higher hazard ratio relative to the Non-D group. Trends for first-hospitalization-free survival rate were similar to those for patient survival.
Discussion
The proportion of participants with falls, frailty, and exhaustion increased as the grade of disability increased. Participants with disabilities had low physical activity. Both univariate and multivariate analyses showed an inverse association between the grade of disability and the PCS or MCS. In addition, SP, EKD, BKD, WS, CF, QSI, sleep, and PS were associated with the grade of disability in both univariate and multivariate analyses. Although the statistical significance was weak, the trend was favorable for the first-hospitalization-free and patient survival rates in the Non-D group. However, there were no significant differences in these rates between the Mild-D and MS-D groups.
Disability was defined as an umbrella term for impairments, activity limitations, and participation restrictions [
2]. Social, medical, or psychological problems lead to functional or intellectual disability, which is associated with inaccessibility to health care services or vulnerability to diseases such as sore or urinary tract infection. Disability can be diagnosed using a medical, functional, or social model, with the functional model (using ADL) being the most commonly used one [
12]. Katz et al. [
13] first published a model using 6 ADLs (bathing, dressing, toileting, transfer, continence, or feeding). Modified scales using 4 or 5 ADLs were introduced after the original version [
6,
14,
15]. We evaluated disability using a model with 4 ADLs [
6].
Dialysis patients are at a high risk of falls and these are associated with adverse outcomes. In addition, previous studies have shown an association between frailty/exhaustion and cardiovascular disease or mortality, in dialysis and nondialysis populations [
16,
17]. Therefore, we aimed to identify the association between disability and these variables. First, we analyzed cross-sectional data at enrollment and found that the presence of disability was associated with falls. Although a causal relationship between the two variables was not clear, we suggested that patients with disabilities would be at risk of falls and those who have had falls would be prone to having disabilities. Second, our study showed an association between disability and frailty/exhaustion. Frailty is defined as a medical syndrome with multiple etiologies that is associated with decreased strength, endurance, and physical function [
17]. Sense of exhaustion is defined as a combination of fatigue, lack of energy, feelings of hopelessness, loss of libido, or increased irritability [
16]. In our study, frailty was evaluated based on physical function/performance, VT, and unintentional weight loss. Exhaustion was evaluated using VT component in HRQoL scales. Therefore, frailty focused on physical function and exhaustion focused on subjective sense. Although the two variables closely overlap or correlate, they are different. Our study showed that disability was associated with these two variables (frailty and exhaustion).
Disability was associated with falls, frailty, and exhaustion, all of which can in turn lead to disability; exercise or rehabilitation may be helpful to prevent or decrease these vicious cycles. El-Khoury et al. [
18] performed a meta-analysis for the effect of the exercise program on fall-induced injuries in an elderly population and showed that exercise results in a decrease in all injurious falls. Desai et al. [
19] evaluated the favorable effect of pedaling exercise on a 6-minute walking test and handgrip and pinch strengths in HD patients. A Korean study evaluated the positive effect of intradialytic exercise on daily physical activity, and it was found to improve physical activity [
20]. Additionally, Lorenz et al. [
21] showed a favorable result of a protocolized exercise program in kidney transplant candidates with advanced chronic kidney disease. However, our results showed that exercise decreased as the grade of disability increased. Education and encouragement of active exercise or rehabilitation would lead to improvement and prevention of adverse outcomes in participants with disabilities or those prone to having disabilities.
We evaluated the effect of disability on HRQoL scales in dialysis patients. Jassal et al. [
3] investigated the association between disability scores and HRQoL. However, they evaluated HRQoL using SF-36 alone and only focused on the PCS and MCS among data for the SF-36 scales. In addition, they performed multivariate analyses adjusted by age and country alone. We evaluated HRQoL using the KDQOL-SF and examined the scores for each of the 22 components, including the PCS and MCS. Various covariates were adjusted in our study. Our results showed that all 11 scores in SF-36 including the PCS and MCS were associated with disability, which is consistent with previous study findings. Among kidney disease-specific scales, SP, EKD, BKD, WS, CF, QSI, and sleep were associated with disability. However, SeF, SS, PS, and DSE were not associated with disability; among these, SS, PS, and DSE would be more influenced by social factors such as quality of health care or medical insurance than by medical or functional status in each participant.
A prospective study showed the positive association between the presence of disability and mortality in dialysis patients [
22]. A multicenter study using the Dialysis Outcomes and Practice Pattern Study cohort enrolled 7,226 HD patients and showed that mortality increased and HRQoL decreased as functional dependency increased [
3]. A Japanese study divided the 7,664 HD patients into three groups according to the severity of disability, and a positive association between the grade of disability and early death or all-cause death was observed [
4]. In addition, previous studies showed that multi-domain interventions including nutritional, physical, or cognitive treatment were effective in improving physical or emotional function in the elderly population [
23,
24].
Our results were similar to those from previous studies. This study showed that the Mild-D group had poor patient survival compared with the Non-D group. The MS-D group had poor patient survival compared with the Non-D group, but there was no statistical significance. Additionally, there was no significant difference in patient survival rates between the Mild-D and MS-D groups. The first hospitalization-free survival trend was similar to that of patient survival. The Mild-D group had the poorest survival, possibly because of two factors. First, the use of a subjective method using questionnaires and the lack of consensus for the assessment of the severity of disability are associated with a nonlinear trend between the severity of disability and prognosis. This study did not show poor prognosis in the MS-D group compared to the Mild-D group, and this may be caused by the two aforementioned factors. The presence of disability is associated with high morbidity and mortality. Some studies have shown the association between the severity of disability and prognosis, but there were differences in the methods defining disability and cut-off value for severity [
25,
26]. In addition, Son et al. [
25] showed that the presence of disability was associated with poor prognosis, but prognosis did not differ between mild and severe disability. In our study, disability was defined using questionnaires for ADL, which is a highly subjective method. The patients in the MS-D group had significantly lower scores for body pain, MCS, SP, and QSI than those in the Mild-D group. These revealed that the severity of disability can lead to low HRQoL scores, but measuring the severity of disability using a subjective method can be influenced by low HRQoL scores. Second, the short-term follow-up duration and high patient survival rates in our study may not achieve statistical significance. In addition, the numbers of deaths by CAD, infection, or others, respectively, were 15, 13, and 31 in the Non-D group, four, three, and nine in the Mild-D group, and none, five, and six in the MS-D group. The absolute number of deaths was different among the three groups, but there was no statistically significant difference in the distribution of the cause of death among them (
p=0.271). Further investigations, using a method combined with objective indicators such as handgrip strength or gait speed and data with greater number of deaths during long-term follow-up, would be helpful to identify the difference in prognosis and cause of death according to the severity of disability.
Our study included both HD and PD patients and showed that the number of HD patients decreased as the grade of disability increased. Differences in disability and HRQoL according to modality are additional important issues. There were conflicting results regarding the association between disability/HRQoL and dialysis modality. Although our study did not include these data, a previous study using this cohort evaluated the impact of disability by dialysis modality [
27]. The study showed that disability was more common in PD patients; additionally, MCS and PCS were higher in HD patients than in PD patients.
Malnutrition is associated with a loss of muscle mass, which can lead to disability. Serum albumin and creatinine are well-known indicators of nutritional or muscle mass status, and serum phosphorus is highly associated with protein intake. Therefore, malnourished patients with low serum albumin, creatinine, or phosphorus levels are prone to the development of disability. In our study, the Non-D group had trend for higher serum albumin, creatinine, and phosphorus than the other groups. The trend in these variables would be an extension of the high prevalence of malnutrition in patients with disabilities.
This study has a few limitations. First, our study design was retrospective, and we used a data set from a previous study [
5]. Second, we did not evaluate disability by using questionnaires with traditional measurements, such as those used by Katz et al. [
15]. Questionnaires for five ADLs are most commonly used to define disability [
28]. However, our study evaluated disability using four items (meal, dress/undress, get in or out of bed, and take a bath or shower) among the five to six traditional items. Validity of the use of four items has not been evaluated. However, due to limited effort and time commitments, two similar questions such as those for toileting or bathing can be combined into one question. Third, our study did not include data on dialysis adequacy. Fourth, there was a large difference in the numbers among the groups. The numbers in the Non-D group were greater than those in the other groups. The difference in numbers among the groups is common in studies using diseases with low prevalence and this is associated with selection bias. Studies for two groups may be overcome using statistical methods, such as propensity score matching. However, there was little evidence for propensity score matching methods in studies of three or more groups. Future prospective studies with long-term follow-up and additional parameters such as dialysis adequacy are needed to overcome these limitations.
In conclusion, disability was associated with poorer outcomes, which include falls, frailty, and exhaustion in Korean dialysis patients. Most HRQoL scales and survival trends were poorer in participants with disabilities than those without a disability.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download